Working with Detergents
Detergents can be a sword with two sharp sides. While detergents are excellent for reduction of non-specific binding in co-IP’s and helpful when cells need to be lysed, detergents can be problematic for reversed phase chromatography. In the figure below, a base peak chromatogram is shown (left panel) where a repetitive pattern is observed starting at around 28 minutes. This pattern is typical for detergent or polymer contamination. In the right panel, the corresponding mass spectrum shows ions that differ by 44.0258 Dalton, corresponding to units of ‘CH2CH2O’. If not removed, detergent (and polymer) can impact robustness of the LC experiments and results in signal suppression in the MS experiment.
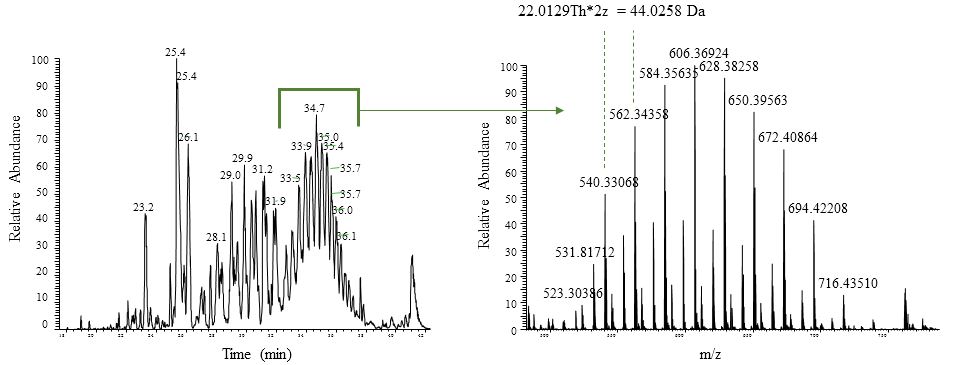
LC chromatogram (left) and MS spectrum (right) of detergent. The mass difference of 42.026 Da corresponds to ‘CH2CH2O’.
Non-ionic detergents exemplified by Triton and NP-40 are relatively easy to remove by 2-3 washes with a salt buffer (for example PBS) free of detergent. Ice cold acetone precipitation (1:6) can also easily remove 0.5% non-ionic detergent. However, SDS is much more resistant to washes and acetone and we suggest to avoiding SDS when possible. Be aware of that a buffer like RIPA does contain SDS.
For some experiments SDS is absolutely needed, for example if the target protein is a membrane related. SDS can be removed by using a Chloroform-Water-Methanol (CWM) precipitation but CWM is less robust when amounts are very limited. Alternatives include S-trap and using SDS-PAGE to remove SDS. The figure below shows how SDS-PAGE can be used to remove SDS. The sample runs 12-15 millimeters into the gel followed by excision and extensive washes of the ‘gel-block’.
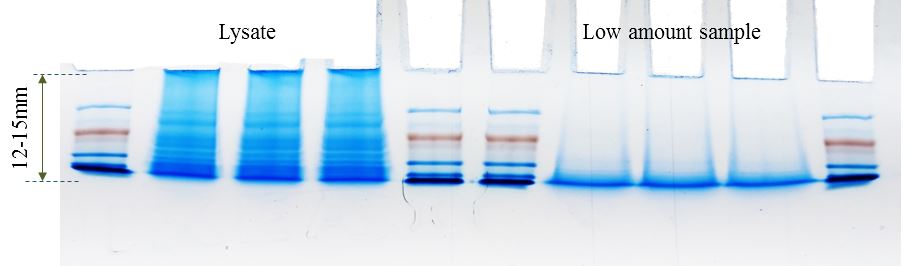
Examples of using SDS-PAGE to cleanup/remove SDS from a sample.