Sweet tooth? Flies have it too—and new research explains how they know what to eat and when to stop
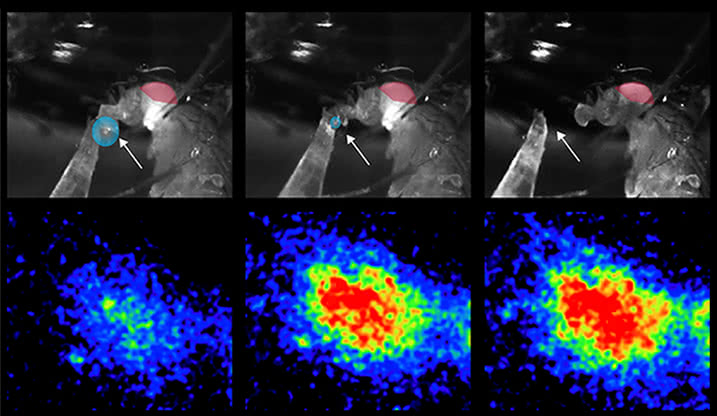
Bottoms up: The researchers filmed hungry flies drinking a drop of sugar solution (blue circles in the top panel) and simultaneously monitored the activity of their IN1 cells before, during, and after the drink was consumed (bottom panels). The cells remained active for several minutes after the solution had been swallowed.
All animals, including humans, love sweet food. But if you’re someone who never turns down dessert under normal circumstances, try wolfing down six donuts as a scientific experiment. Even the moistest, most velvety piece of chocolate cake will seem a lot less appetizing—and you will likely eat less of it.
The brain processes many signals that help us regulate what we eat and how much. How do we know what tastes good and what doesn’t? And how does our brain tell us how much to eat when we’re not really hungry, versus when we’re famished after a long workout?
Researchers at The Rockefeller University working with Drosophila flies have brought us one step closer to understanding the biology of eating. In a recent study published in Cell, they’ve identified a set of neurons that are activated only when flies eat a very sweet solution—especially when the flies are hungry. If the food is less sweet, or when the flies are relatively full, these neurons become less active.
The researchers were surprised to discover that these brain cells connect to taste neurons in the pharynx, or throat, rather than in the fly’s equivalent of the tongue—which means flies can directly taste and monitor food while swallowing it.
“These neurons in the fly brain are part of something akin to a ‘food circuit,’” says Nilay Yapici, a postdoctoral fellow in the lab of lead author Leslie Vosshall, Robin Chemers Neustein Professor and head of the Laboratory of Neurogenetics and Behavior as well as associate director of the Kavli Neural Systems Institute at Rockefeller. Some aspects of this food circuit exist in many other animals, such as mice and humans. One of the next steps in the research will be to examine whether the specific neurons Yapici and Vosshall have identified in flies exist in the mammalian brain. “We still don’t know if that’s the case,” Yapici says, “but it would be very exciting, especially if it enables us to learn more about how we eat—and why we often eat too much.”
Dinner time
Here’s how the circuit appears to work: Taste neurons in the flies’ pharynx (throat) connect to a group of 12 neurons, known as IN1 cells, which in turn transmit signals to the neural circuits that tell the brain whether to keep eating.
“These 12 interneurons help the brain identify what the flies are eating, and help regulate whether to continue or stop,” says Yapici. “If we give the flies something sweet and they are hungry, they will eat continuously. If it’s less sweet, they don’t eat as much. The neurons are helping the brain evaluate what the animal’s eating while it’s eating it.”
It’s hard to track how much flies eat every day. Each fly is tiny, and consumes roughly a microliter of food daily, making it very difficult to measure slight differences in food intake. For the current study, Yapici, Vosshall, and their colleagues pioneered a new technique they call Expresso, an exquisitely precise sensor that continuously records how much the flies are consuming in real time.
While the flies are eating, the researchers can observe their brains using a monitor that captures calcium levels in neurons, a proxy for neuronal activity. This part of the study was done in collaboration with Raphael Cohn, a graduate student in the laboratory of Vanessa Ruta, Gabrielle H. Reem and Herbert J. Kayden Assistant Professor and head of the Laboratory of Neurophysiology and Behavior.
To identify the specific neurons involved in eating behavior, the researchers inhibited different populations of neurons, and watched what changed as a result. They found when they inhibited the IN1 cells, the flies started to eat but would stop prematurely, even if they were still hungry. “Silencing the activity of these neurons appears to suppress food intake,” says Yapici. What’s more, when the researchers turned these neurons back on, satiated flies ate as if they were starving.
Next, the researchers observed how this specific group of neurons behaves under normal situations. They found that when hungry flies drink even a tiny amount of tasty, sweet food, the IN1 cells become activated and remain active for many minutes after the food has been swallowed. The researchers think that the activity of the IN1 cells drives these animals to ingest food. So it makes sense that when the flies aren’t hungry and encounter sweet food, the neurons still become active, but quiet down relatively quickly.
When the flies are hungry and only have the option of less tasty food, the neurons still exhibit a burst of activity, but it quickly quiets down, similarly to what happens when satiated flies are given tasty food. In each case, the activity of IN1 cells mirrored the eating behavior of the fly.
From insects to mammals
The researchers believe these findings may have implications for diseases related to food intake such as obesity. “The goal of studying food intake behavior,” says Yapici, “is to understand the biological signals that make us eat.”
By working with flies, which have relatively small brains compared to mammals, the researchers can more easily identify and manipulate specific circuits that regulate food intake, then see if similar pathways are at play in animals with more complex neurocircuitry, such as mice and other mammals. “If you find a neural mechanisms in the fly, you can look for similar principles in a mouse model—since you know what you are looking for, it may be easier to find,” says Yapici.
 Cell, online: March 31, 2016 Cell, online: March 31, 2016A taste circuit that regulates ingestion by integrating food and hunger signals Nilay Yapici, Raphael Cohn, Christian Schusterreiter, Vanessa Ruta, and Leslie B Vosshall |