Study reveals the structure of a protein crucial for DNA replication
Our DNA contains instructions crucial for virtually all forms of life on this planet. But for life to propagate, this blueprint must be copied and passed on to future generations. New findings describing the structure of a key molecule involved in DNA replication place another piece in the puzzle of how this process works.
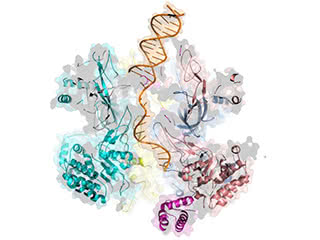
Take your places: The structure unveiled that part of the helicase known as the N-tier travels ahead of the C-tier along the leading DNA strand, reversing previously held assumptions about how the replication machinery is oriented.
“Biologists have learned a great deal about the molecular structure and functions of the enzymes and proteins related to DNA replication,” says O’Donnell, head of the Laboratory of DNA Replication. “However, we still have much to discover, and this understanding of helicase activity brings us another step closer.”The latest research from Rockefeller’s Michael O’Donnell, Anthony and Judith Evnin Professor and Howard Hughes Medical Institute investigator, and Huilin Li, professor at the Van Andel Research Institute, elucidates the interaction between DNA and a protein called CMG helicase, whose function is to open up the DNA double helix like the slider of a zipper, preparing its genetic code for copying. The study published recently in the Proceedings of the National Academy of Sciences.
Findings from the new study reverse a long-held assumption about how the helicase protein interfaces with DNA. Images taken during DNA unwinding demonstrate that its orientation is opposite from what scientists have reported previously, and these findings have important implications for understanding how the replication process works.
“Since discovery of the DNA double helix more than 50 years ago, helicase’s activity in preparing DNA for replication has been poorly understood,” says Li. “However, recent advances in microscopy and study design allow us to create accurate images of these enzymes and observe their interactions with DNA for the first time.”
The structure of the helicase on DNA was derived at Rockefeller University’s cryo-electron microscopy core facility, leveraging a groundbreaking imaging technology that has revolutionized scientists’ ability to visualize and understand the role of fundamental biological processes.
More than 40 diseases—including many cancers and blood and muscle disorders— trace their origins at least partially to inaccuracies or failures in DNA replication. O’Donnell and Li hope the results of this study will eventually help the development of new treatments for these diseases.



