New molecular map reveals how cells spew out potassium
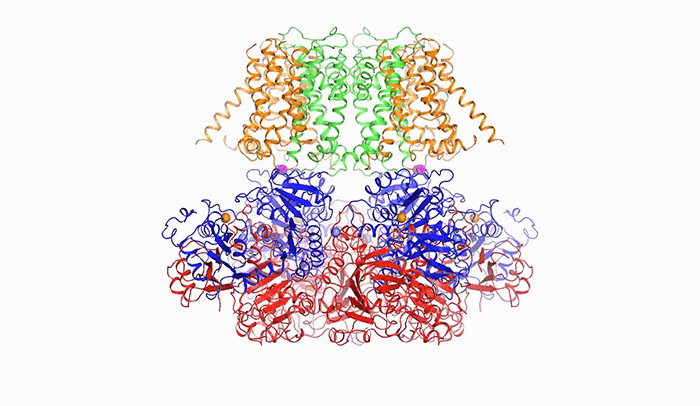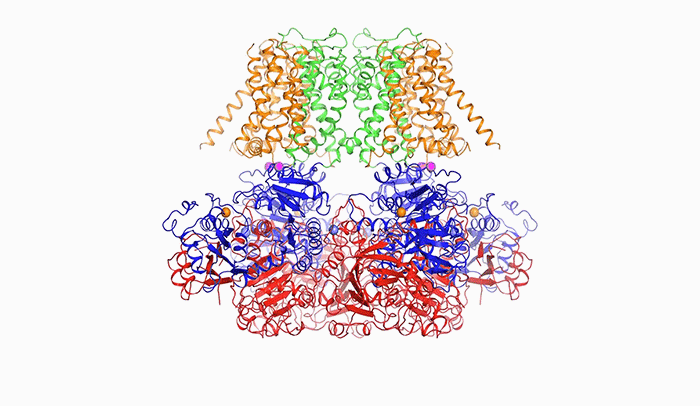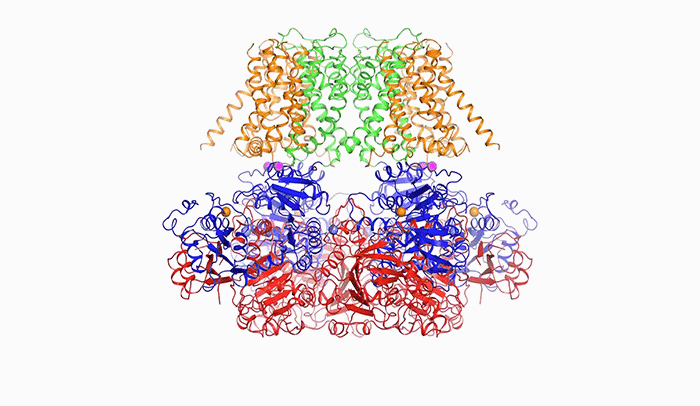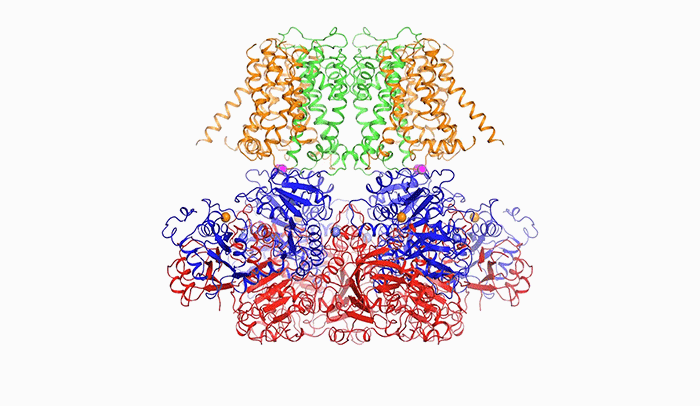
Open sesame: In order to release potassium, BK is prompted to open by calcium (above), a change in voltage, or a combination of the two.
New research from Roderick MacKinnon’s Laboratory of Molecular Neurobiology and Biophysics at The Rockefeller University has determined, for the first time, the complete structure of an ion channel that plays an important role in cellular electrical signaling by sending potassium ions out of the cell at an extremely rapid rate.
By revealing new insights into how the molecule works, this research leads to a deeper understanding of the link between the membrane and processes inside the cell, including calcium regulation of electrical signals, which is central to muscle contraction and neural activity. The results are described in two papers published in Nature on December 14.
Potassium channels both regulate the occurrence of electrical impulses and terminate the impulses once they are generated. One such potassium channel, known as the BK or “big potassium” channel, conducts ions up to a level 20 times that of other potassium channels. To do so, BK responds to two separate triggers—electrical activity on the cell membrane and levels of calcium—that it ties together.
When BK malfunctions, cells can become too active because they can’t turn off their electrical impulses. This contributes to diseases such as hypertension, a hereditary form of asthma, and overactive bladder, in which smooth muscles in the vascular system, airway, or bladder are overactive.
No need for crystals
MacKinnon began trying to work out the structure of this channel, including the tunnel-like pore through which the ions travel, about 15 years ago; but new imaging technology has only just now made this effort feasible. That’s because for decades, scientists typically had to grow crystals out of protein molecules in order to determine their structures. However, as is often the case with large proteins, BK would not cooperate, and scientists were not able to turn it into crystals.
Thanks to a recently developed approach known as cryo-electron microscopy, however, researchers like MacKinnon no longer need crystals. Instead, they can freeze molecules in ice. Using state-of-the-art equipment installed at the Evelyn Gruss Lipper Cryo-Electron Microscopy Resource Center, MacKinnon, research associate Xiao Tao, and postdoc Richard Hite created a three-dimensional reconstruction of the arrangement of atoms within BK. That atomic-scale molecular structure helps explain how BK is able to disgorge so much potassium compared with other ion channels.
Molecular secrets revealed
“The pore within BK is much wider than those of other potassium channels. In fact, it forms a wide funnel that opens up to the interior of the cell,” Tao says. “And the funnel’s surface bears a negative charge, which attracts positively charged potassium ions from within the cell toward the pore.”
In order for potassium to flow through BK, the channel’s pore must open, and it does so in response to two triggers: calcium, another ion important to cellular signaling; and a change in voltage across the cellular membrane, which occurs when a cell generates an electrical impulse.
After binding to calcium ions, the channel compresses itself, changing shape in such a way that its pieces are pulled outward and the pore widens.
While the researchers could not directly observe how the channel responds to a change in voltage, the structure did provide clues to explain this opening mechanism. Sensitivity to both calcium and voltage most likely allows the channel to fine-tune its responses, the researchers say.
“Researchers have been looking to treat a number of conditions by developing drugs to activate BK, but without success so far,” Hite says. “With the atomic structure of BK, we now have a map to guide these efforts by helping us understand which parts of the channel could be most effectively targeted.”
MacKinnon began studying the functional properties of this channel as a postdoc. “BK is the first potassium channel I studied, so it is enormously satisfying to finally observe its molecular structure,” says MacKinnon, who is the John D. Rockefeller Jr. Professor, a Howard Hughes Medical Institute investigator, and a recipient of the 2003 Nobel Prize in Chemistry.
This work was supported by the National Institutes of Health (grant GM43949).
 Nature online: December 14, 2016 Nature online: December 14, 2016Cryo-EM structure of the open high-conductance Ca2+-activated K+ channel Xiao Tao, Richard K. Hite, and Roderick MacKinnon |
 Nature online: December 14, 2016 Nature online: December 14, 2016Structural basis for gating the high-conductance Ca2+-activated K+ channel Richard K. Hite, Xiao Tao, and Roderick MacKinnon |


