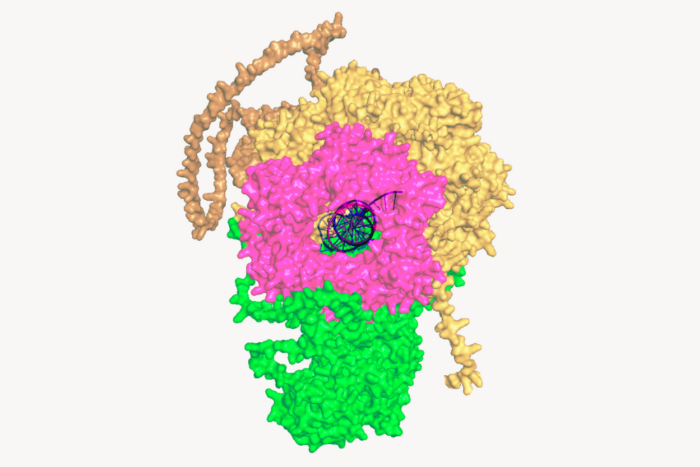
What these scientists revealed about the structure of the T cell receptor could help resolve a decades-old debate over how T cells get activated

Ryan Notti (left) and Thomas Walz (right) discovered new characteristics of a T cell receptor that are essential to a variety of cutting-edge T cell immunotherapies.
Adoptive T cell therapies—in which T cells are re-engineered outside the body to recognize dangerous cells and then infused back into the body to go on the attack—are on the frontier of medicine. One such approach, using chimeric antigen receptor, or CAR-T, cells, has shown remarkable efficacy for the treatment of liquid tumors (which form in blood, lymphatic fluids, or bone marrow), with many patients experiencing tumor reduction or long-term remission. However, it has yielded much more disappointing results in the solid tumors that account for most cancers, with response rates below 25%. And no one has really known why.
Ryan Notti, a special fellow in the Department of Medicine at Memorial Sloan Kettering Cancer Center and an instructor of clinical investigation in Thomas Walz’s Laboratory of Molecular Electron Microscopy at Rockefeller University, teamed up with Walz to see whether clues might lie in the T cell receptor’s fundamental structure.
As they published in Nature Communications, they used cryo-EM to discover characteristics of the T cell receptor (TCR)—essential to a variety of T cell therapies—that had never been observed before. “All the data we’d read depicted TCR as being open and extended in its dormant state, but we found that before activation, it has a compacted, closed shape,” Notti says. “After binding to an antigen, it sort of springs open like a jack-in-the-box.”
This work was made possible in part by Rockefeller’s Clinical Scholars Program, a three-year program designed for clinically trained medical professionals to get laboratory research training and learn the ins and outs of translational research. Most graduates of this program are physician-scientists like Notti who work at the intersection of medical practice and laboratory research, bringing insights from patient care to the lab and vice versa.
We spoke to Notti and Walz about the translational potential of this partnership of clinical insight and basic research, from new cancer treatments to improved vaccine design.
How did this line of research develop?
Ryan Notti: I initially had the idea when I was doing my medical residency in 2018. One of the big questions I was interested in was how these T cell therapies actually work at the molecular level, and I couldn’t get a good answer to how a TCR or a CAR gets activated. Also, these therapies don’t work for most cancers, and understanding exactly why that is, and how to fix that, is hard without a fundamental understanding of how these biological machines work. I had gotten my Ph.D. in structural microbiology from Rockefeller in 2015, so I thought basic research of the T cell receptor’s structure, particularly in a membrane environment, could potentially provide this knowledge. Tom is a luminary in the field of electron microscopy—especially when it comes to imaging challenging membrane proteins—so when he said he was interested in the project, I was thrilled. Working in Tom’s lab then dovetailed nicely with my joining the Clinical Scholars program, which I did because I wanted to further my knowledge of translational science and ensure protected research time.
Thomas Walz: When Ryan suggested it, I was thrilled as well. It’s very rare to have a medical doctor who is interested in structure, and the project was right in line with my lab’s basic research on membrane proteins. The TCR is embedded in the T cell membrane, and my group specializes on studying membrane proteins in custom-designed membrane environments. We can change things like the thickness of the membrane, the size—all kinds of membrane parameters—and then capture how proteins react to them using cryo-EM.
I also thought it was a great project because it shows the strengths of both basic research and the translation of it. A better knowledge of almost any protein will have a biomedical application at some point.
And what did your work reveal about the process of T cell activation?
RN: How it gets activated has been a major debate for 40 years—ever since the T cell receptor was first discovered—and whether it undergoes a shape change as a result. We found that it does, which helps to explain how information gets from outside the cell, where antigens such as infectious agents or proteins from a malignancy are being presented to the receptor, to the inside of the cell, where the signaling happens that turns on the T cell.
TW: That the basis of T cell signaling has remained so controversial until our work is quite remarkable. And there are more foundational discoveries about the TCR to come. In this paper, we published two of TCR’s conformational states, but Ryan has already imaged many, many more.
What are the health implications of your findings?
RN: For one thing, we anticipate that this work will be useful for re-engineering receptor-based and cell-based cancer therapies. For example, my clinical specialty is sarcomas—cancers that arise from soft tissue or bone—and adoptive T cell therapy has been used successfully for certain very rare sarcomas. Perhaps these insights can help fine tune receptor sensitivity to make this approach more effective for a wider range of sarcomas.
It also may help improve vaccine design. Folks in the field can use our structures to see which types of antigens might be better or worse at activating the TCR, and whether these different modes of interaction have implications for how the receptor functions. Understanding how the TCR responds to foreign antigens is important for vaccine design, because one of the functions of T cells is to signal to B cells, which produce antibodies. Getting T cells and B cells to talk to each other is an important part of making an effective vaccine.
More generally speaking, I think our study is a great example of how basic science is essential for accelerating improvements in the clinical space.
TW: Yes, exactly. If no one is pursuing the kind of basic research we conduct at Rockefeller, there will be nothing to translate in the future.