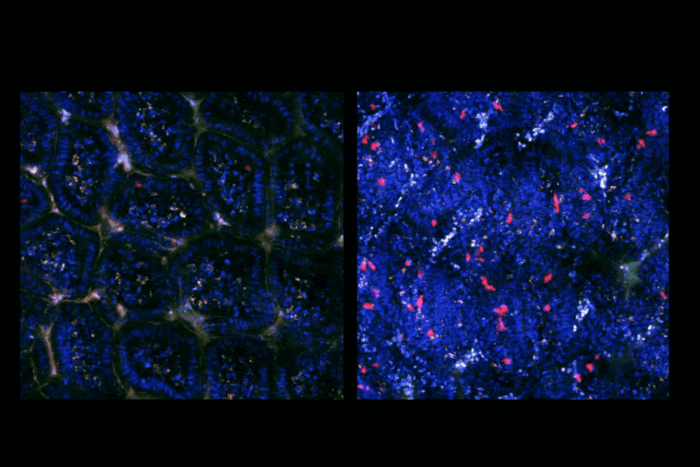
Microbes in the gut may influence metabolism
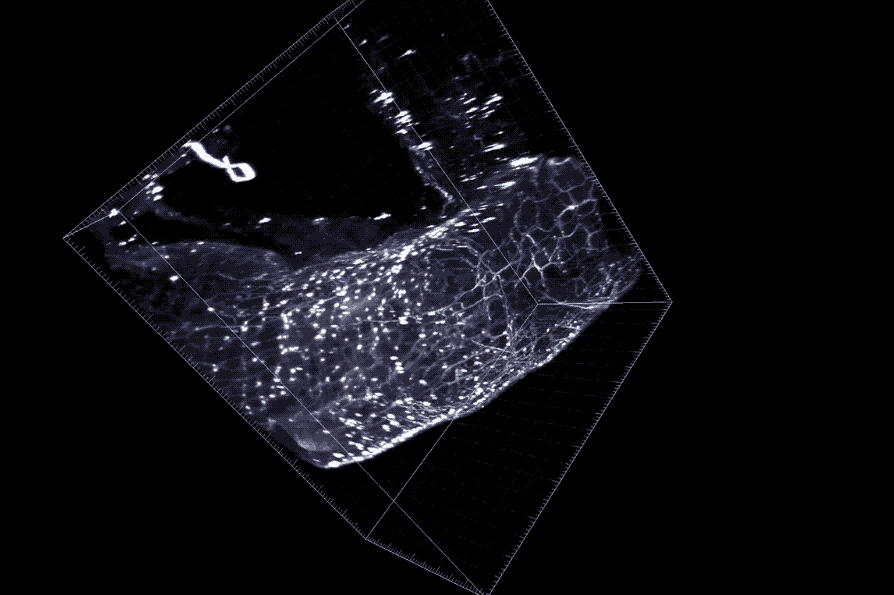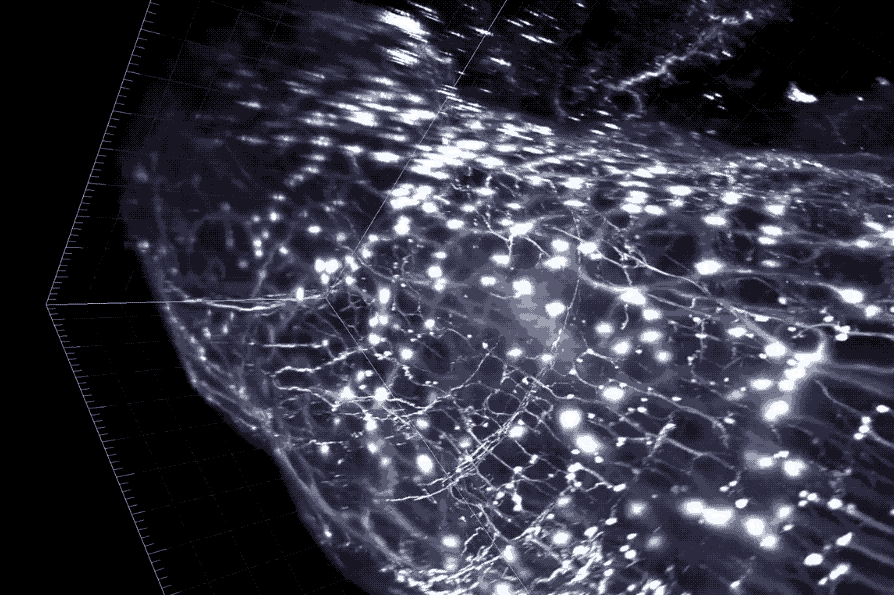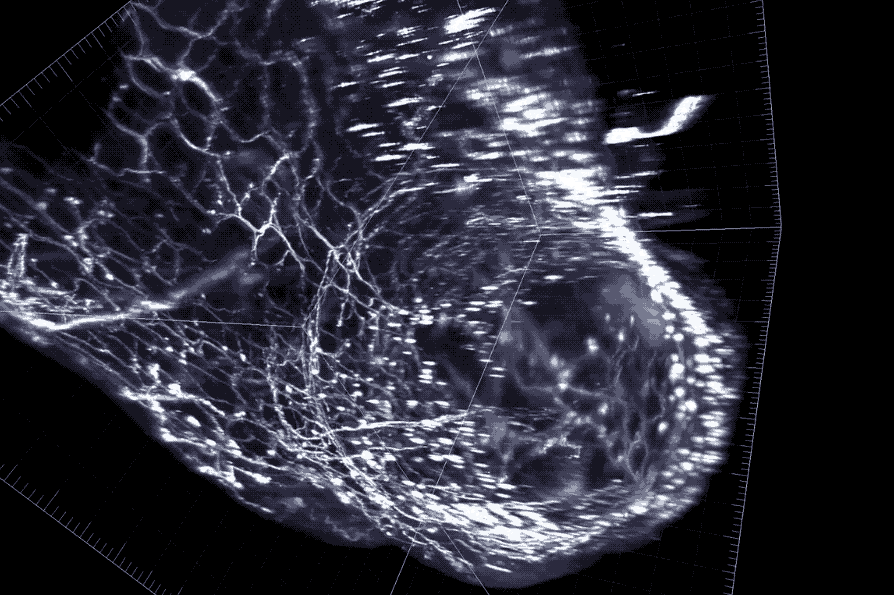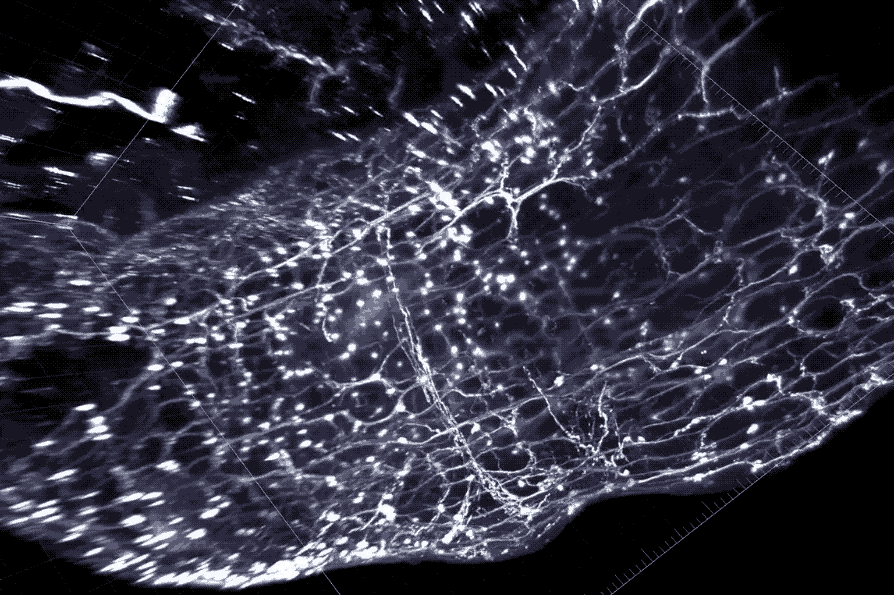
A cross section of the colon with enteric neurons highlighted. These neurons interface directly with gut microbes.
The 10 trillion bacteria living in your digestive system may not be human, but they seem to be as integral to your body as your heart or liver. A growing number of studies are finding that microbes in the gut directly influence biological processes from bowel movements to behavior.
New research in mice now reveals that these microbes may shape our metabolism, as well. Researchers in Daniel Mucida’s Laboratory of Mucosal Immunology have identified a particular type of gut neuron that controls blood sugar levels, influencing appetite. Published recently in Science, the findings could have implications for treating metabolic disorders such as obesity and diabetes, which are closely related to levels of glucose in the blood and have been linked to changes in the composition of the gut microbiota.
“We know that microbes in the gut can produce metabolites that mimic neurotransmitters and are detected by enteric neurons. Now we are beginning to find direct connections between microbial activity and host behavior, such as eating. It’s a remarkable role for these organisms,” says Mucida.
The work was led by Paul Muller, a recent graduate student in Mucida’s laboratory, Fanny Matheis, another Mucida graduate student, and Marc Schneeberger Pané a postdoctoral fellow in Jeffrey Friedman’s Laboratory of Molecular Genetics.
Geography matters
The gut is enmeshed by neurons—to the extent that it may be considered a nervous system in its own right, so sophisticated it is sometimes referred to as our second brain. These neurons help with digestion and motility by keeping close tabs on a variety of molecular cues, most of which are thought to come either from our diet or from microbes that call the gut home.
But these microbes are not distributed uniformly throughout the digestive tract. The initial segment, known as the duodenum, harbors relatively few microbes, whereas the end, the colon, contains the vast majority. Mucida and colleagues have found that the same is true for gut neurons, and an analysis based on the types of proteins made in each segment of the mouse gut has revealed that neurons in fact perform different functions depending on where they’re located.
But in germ-free mice—bred under sterile conditions to prevent colonization with microbes—the gradient was diminished: The quantity of neurons in regions higher in microbes resembled that of the microbially-scarce duodenum under these circumstances. These findings suggest that microbes are, in large part, responsible for the neurons’ regional differences.
To test this idea, Mucida’s team treated mice with antibiotics, and discovered that by reducing the number of bacteria they also caused a drop in the number of neurons throughout the gut—an effect that eventually went away after the antibiotics were stopped. Neurons located near the end of the intestines were hit particularly hard by the treatment, suggesting that they may be especially reliant on microbes. Experiments also showed that this loss of neurons was mediated by neuronal expression of an inflammatory sensory pathway previously shown to control loss of neurons during intestinal infection.
A link to sugar metabolism
But the researchers still did not know what roles these microbe-regulated neurons play in the body. So, they engineered the mice in such a way that they could selectively manipulate the subset of neurons in the ileum and colon at will. To their surprise, they saw that the mice’s appetites decreased when the neurons were activated, while their blood sugar level increased. And deleting the same neurons had the opposite effect.
In mice that did lose this subset of neurons, there was no change in blood sugar levels in response to antibiotics. “It was totally unexpected to find that only controlling a small proportion of neurons in the gut could have such a dramatic impact on metabolism in the mouse,” says Muller.
“This mechanism of regulating blood glucose seems to act independently of the brain, but rather by a direct line of communication between gut, pancreas and liver—a finding with potential therapeutic implications for metabolic diseases,” says Matheis.