Scientists engineer human-germ hybrid molecules to attack drug-resistant bacteria
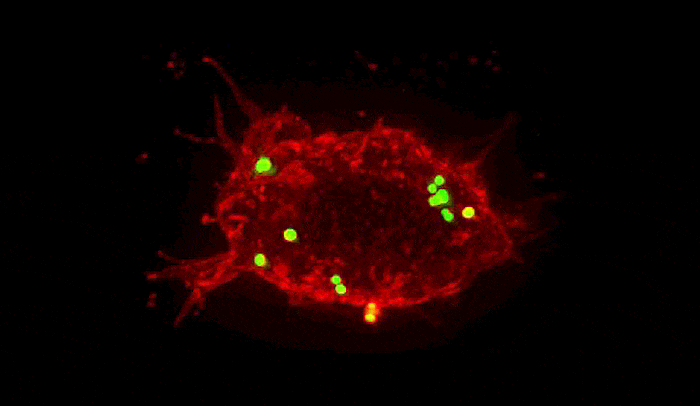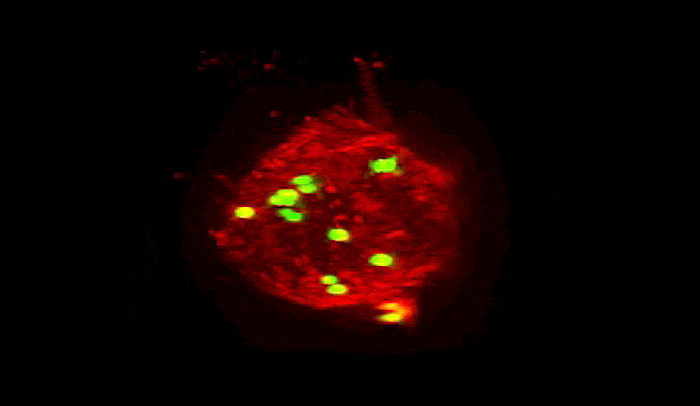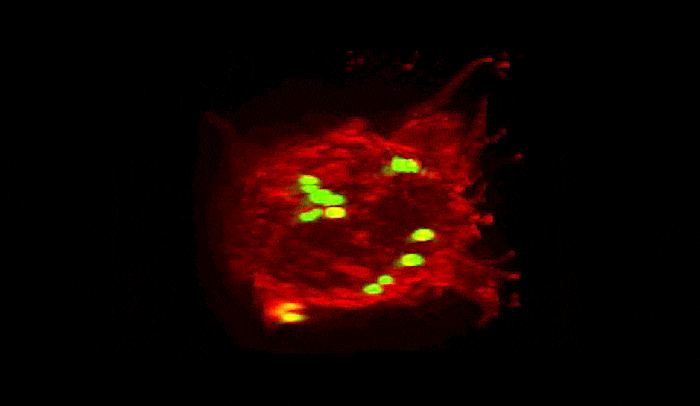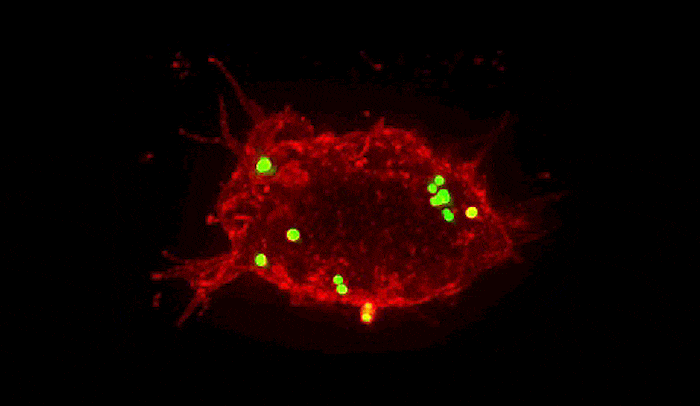
Summoned by lysibody molecules, large immune cells called macrophages (red) engulf Staphylococcus aureus bacteria (green).
Inspired by viruses that attack and kill bacteria, researchers at The Rockefeller University have created an entirely new weapon against disease-causing bacteria that shows great promise for treating drug-resistant infections.
In work described in the Proceedings for the National Academy of Sciences on April 17, the team engineered molecules that accomplish something viruses do much better than the human immune system; namely, targeting specific carbohydrate molecules that appear on the surfaces of bacterial cells.
“Bacteria-infecting viruses have molecules that recognize and tightly bind to these common components of the bacterial cell’s surface that the human immune system largely misses. We have co-opted these molecules, and we’ve put them to work helping the human immune system fight off microbial pathogens,” says Vincent A. Fischetti, head of the Laboratory of Bacterial Pathogenesis and Immunology.
In experiments with mice, Fischetti’s team used this approach to successfully treat life-threatening infections by MRSA, a bacterium that is resistant to conventional antibiotics—results that suggest they may have found a new way to fight superbugs like MRSA.
The enemy of my enemy
Just as disease-causing bacteria seek to infect us, some viruses prey upon bacteria. And these viral predators have developed a keen ability to latch onto and cut through the outer surface or walls of the bacteria, killing the cells in the process. They do so using molecular snippers called lysins that bind to specific carbohydrates in cell walls.
The human immune system, meanwhile, has a blind spot for carbs. It produces antibodies that are particularly good at binding to proteins on bacterial cell walls, thereby tagging the bacteria for destruction by immune cells. But when their target is a carbohydrate, not a protein, the human antibodies fall short.
Even so, lysins and antibodies share some similarities in their structures. And that gave the researchers an idea.
“Both antibodies and lysins have two discrete components. They both have a part that binds their respective target, but whereas the second component of lysins cuts the bacterial cell wall, in antibodies it coordinates an immune response,” says Assaf Raz, a research associate in Fischetti’s lab who led the experiments. “This made it possible for us to mix and match, combining the viral piece responsible for latching onto a carbohydrate with the part of the antibody that tells immune cells how to respond.”
The team also looked to bacteria themselves: Like viruses, bacteria produce a similar carbohydrate-binding and cutting molecule, which they use to alter their own cell walls during growth. As they did with the lysins, the researchers combined the binding region from one of these remodeling enzymes with a piece of human antibody.
Lysibodies in action
The researchers dubbed their creation “lysibodies,” and made three types: two derived from viruses, one from bacteria. All were designed to kill Staphylococcus aureus, a common bacterium responsible for everything from minor skin infections to pneumonia and meningitis. While many of these infections can be treated with antibiotics, the emergence of strains of drug-resistant Staph, including one known as MRSA, has created a need for a new way to fight these pathogens.
In experiments, the team found that, as they had hoped, the lysibodies attached to the carbohydrates on the surface of Staph and induced immune cells to engulf and destroy them. Since multiple types of related bacteria can have the same carbohydrate targets, the lysibodies grabbed onto a variety of strains of Staph. As an added benefit, one even latched onto more far-flung relatives, including the bacteria responsible for strep throat and urinary tract infections.
The lysibodies further proved their mettle by thwarting MRSA. Treatment with one lysibody greatly improved the survival of MRSA-infected mice, while treatment with another prevented severe kidney infections in the rodents.
Staph and beyond
The process of testing lysibodies’ potential to fight MRSA and other dangerous Staph infections in humans has already begun. The Tri-Institutional Therapeutics Discovery Institute, a partnership established to expedite early-stage drug discovery, is manufacturing lysibodies and has plans to begin testing their safety.
Since nearly all bacteria may be infected by lysin-producing viruses, lysibodies could be produced against many disease bacteria. Furthermore, “based on our results, it may be possible to use not just lysins, but any molecule with a high affinity toward a target on any pathogen—be it virus, parasite, or fungus—to create hybrid antibodies,” Fischetti says. “This approach could make it possible to develop a new class of immune boosting therapies for infectious diseases.”