Mutations in a single gene underlie vulnerability to two unrelated types of infections
When a genetic error weakens a child’s immunity, otherwise nonthreatening microbes can sicken and sometimes kill. In work published July 9 in Science, researchers at The Rockefeller University and their colleagues identify one surprising case in which mutations in a single gene render children vulnerable to two very different diseases: aggravating, but treatable fungal infections, as well as invasive and potentially fatal bacterial disease.

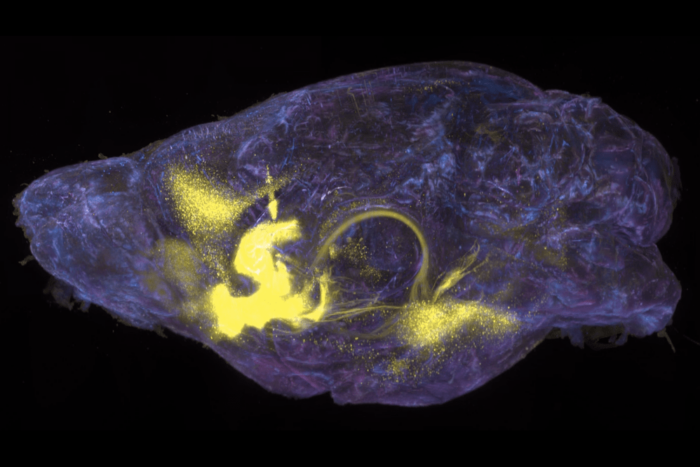
Dangerous vulnerability: Researchers have identified a new immune system deficiency in which mutations in the gene RORC result in susceptibility to otherwise relatively harmless mycobacteria. A six-year-old Chilean girl with RORC defects had mycobacterial abscesses (white) in her brain and abdomen.
For the last 20 years, Rockefeller’s Jean-Laurent Casanova, professor, head of the St. Giles Laboratory of Human Genetics and Infectious Disease, Howard Hughes Medical Institute investigator, and a senior attending physician at The Rockefeller University Hospital, has sought to identify the genetic errors responsible for selective vulnerability to certain infections, including those caused by so-called mycobacteria, a group of pathogens responsible for well known diseases such as tuberculosis.
In this case, the mutations they found in samples from one patient, a Palestinian child, with disease caused by the tuberculosis live vaccine didn’t seem to make sense at first. These defects, in an immune gene known as RORC, had previously been linked in experimental infections in mice to chronic yeast infections. Follow up work, including samples from other children in the same family, and others in Chile and Saudi Arabia, confirmed, however, that defects in RORC also bore responsibility for mycobacterial diseases, caused by the tuberculosis vaccine in six children and by the agent of tuberculosis itself in another.
“Over the years, my lab has found that inborn errors in the production of an immune system signaling molecule, or cytokine, called interferon-γ specifically impair the defenses against relatively harmless types of mycobacteria, such as the live tuberculosis vaccine,” Casanova says. “Our experiments have shown that mutations in RORC give rise to multiple problems within the immune system, including interfering with the production of interferon-γ, which is crucial for fending off mycobacteria.”
While the highest profile members of this group include the microbes responsible for tuberculosis and leprosy, other mycobacteria are weak threats at best. Inherited problems with interferon-γ render the patients vulnerable to this latter group, including live bacilli Calmette-Guerin (BCG) vaccines used to inoculate against tuberculosis. Indeed, for people who carry rare genetic defects, the BCG vaccine can cause severe, even fatal illness. Casanova works with physicians from around the world to collect samples from patients who suffer from BCG and other mycobacterial infections. His lab then searches these patients’ genetic sequences for the mutations responsible for this vulnerability.
While examining the genome of the Palestinian child, one of the first authors, Satoshi Okada, then a postdoc in the lab and now an assistant professor of pediatrics at Hiroshima University Graduate School of Biomedical & Health Sciences, found that this child carried two mutated copies of RORC. RORC was known to control the production of cytokines called interleukin-17 by T cells, which are important in mice and humans for host defense against yeasts known as Candida, which infect the skin, nails, and mucous membranes. Indeed, the lab had previously shown that inborn errors of interleukin-17 immunity underlie chronic mucocutaneous candidiasis. Work in mice revealed why this happens: The protein encoded by RORC helps control the development of interleukin-17-producing T cells.
“The RORC mutations seemed to explain the child’s thrush, an oral fungal infection, but I was skeptical that these could be linked to the BCG infection that ultimately caused her death. However, after two other children in her extended family contracted similar infections, I found sets of RORC mutations in each, strongly suggesting a connection to the illnesses,” Okada says.
To understand the genetics of the disease, it is important to note that the Palestinian children’s parents were first cousins. Such a relationship between parents puts their children at a high risk of inheriting two defective copies of the same gene, and as a result, suffering from extremely rare disorders.
The link between RORC and the mycobacterial vulnerability was confirmed in the genomes of one Chilean and three Saudi children, also the offspring of consanguineous marriages who suffered from a similar combination of diseases. Okada set about doing molecular experiments to assess the mutant proteins produced by the defective RORC genes. The other first author, Janet Markle, a postdoc in the lab, later took on this work, and sought to link these molecular changes with the patients’ vulnerabilities to disease. Collaborators in Australia, Switzerland, and Boston contributed to the experiments.
In these new experiments, the researchers verified that the RORC mutations found in the patients dramatically decreased their ability to produce interleukin-17. They also pinned down a connection to interferon-γ, and as a result, mycobacterial disease.
“When looking at the immunological effects of the RORC mutations, we found a variety of problems, which appear to culminate in the impairment in T cells’ ability to produce interferon-γ in response to mycobacteria,” Markle says. “Although we don’t yet know the details of how this happens, it is clear that RORC plays a crucial role in the immune response to these bacteria.”
“While Candida infections can be treated with anti-fungal medications, mycobacterial disease can be life-threatening. Our results show that patients who suffer from mycobacterial infections as a result of RORC mutations can be treated with interferon-γ — a potentially life-saving intervention,” Casanova says. “By finding that RORC helps control the development of these two cytokines, interleukin-17 and interferon-γ, we have revealed a surprisingly dual role for RORC in human immunity to infection.”
 |
Science: online July 9, 2015 Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations Satoshi Okada, Janet G. Markle, Elissa K. Deenick, Federico Mele, Dina Averbuch, Macarena Lagos, Mohammed Alzahrani, Saleh Al-Muhsen, Rabih Halwani, Cindy S. Ma, Natalie Wong, Claire Soudais, Lauren A. Henderson, Hiyam Marzouqa,Jamal Shamma, Marcela Gonzalez, Rubén Martinez-Barricarte, Chizuru Okada, Danielle T. Avery, Daniela Latorre, Caroline Deswarte, Fabienne Jabot-Hanin, Egidio Torrado, Jeffrey Fountain, Aziz Belkadi, Yuval Itan, Bertrand Boisson, Mélanie Migaud, Cecilia S. Lindestam Arlehamn, Alessandro Sette, Sylvain Breton, James McCluskey, Jamie Rossjohn, Jean-Pierre de Villartay, Despina Moshous,Sophie Hambleton, Sylvain Latour, Peter D. Arkwright, Capucine Picard, Olivier Lantz. Dan Engelhard, Masao Kobayashi, Laurent Abel, Andrea M. Cooper, Luigi D. Notarangelo, Stéphanie Boisson-Dupuis, Anne Puel, Federica Sallusto, Jacinta Bustamante, Stuart G. Tangye, and Jean-Laurent Casanova |