The protein's in the mail
New findings from Blobel lab enhance understanding of “ZIP Code” protein transport system

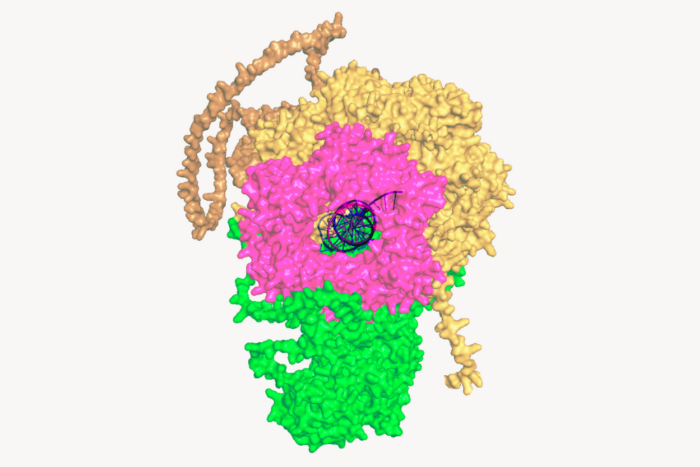
A "skeletal" view of the part of the SRP receptor visualized by Schwartz and Blobel. The two protein subunits bind each other only when the beta subunit (left), a molecular switch, is in the “on” position. "On" and "off" states are defined by the position of the switch loop (yellow) in the center of the protein complex. In the “off” position the switch loop changes its shape, and hence the subunits fall apart. Before this study, it was widely believed that the SRP receptor subunits were permanently associated in the cell.
A busy urban post office daily sorts thousands of letters and parcels, guiding each to a particular mailbox somewhere in the city. Each day, every cell of the human body manufactures millions of proteins which it also must continually sort and route to their final destinations within the cell. Only when a protein has reached its destination can it do its assigned work.
But just how do the proteins get where they need to go? Rockefeller University professor Günter Blobel, an investigator at the Howard Hughes Medical Institute, won the 1999 Nobel Prize in Physiology or Medicine for discovering that each cell uses a “ZIP Code”-like system to shuttle proteins to their intended destinations. Now, new research from Blobel’s Laboratory of Cell Biology, reported in the March 21 issue of Cell, provides a more detailed picture of the “sorting” mechanism used in the cell’s “post office.”
Using a technique known as X-ray crystallography, Thomas Schwartz, postdoctoral associate in the Blobel lab and first author of the Cell, paper, has solved the structure of the SRP (for Signal Recognition Particle) receptor. “This structure illuminates the mechanics of an important sorting mechanism, whose role has up to now been poorly understood, in the eukaryotic cell,” says Blobel, senior author of the paper.
The SRP receptor is a heterodimer or functional complex formed when two molecular switches are bound together. (A molecular switch, like a light switch, is a molecule whose “on” and “off” positions correspond to different respective effects on cell function.) Schwartz’s structure demonstrates for the first time that two switches, called SR alpha and SR beta, exist independent of one another in the cell. They come together and form the SRP receptor only as needed to shuttle certain kinds of proteins to their destinations, separating again afterwards.
These findings, which furnish scientists who focus on protein transport with a more precise understanding of the ZIP Code system, are relevant to understanding the origins of many inherited diseases — including, for example, cystic fibrosis — that arise when proteins are “mislocalized” (sent to the wrong location in the cell) as a result of errors in the cell signaling that routes proteins to their intended destinations.
At an even more fundamental level, misfirings in the protein-transport system, known as protein secretion, “are intolerable to the cell,” says Schwartz. “It is essentially impossible for the cell to survive major errors in this process, which occurs at the most basic level necessary for survival.”
Switch campaign
The life of a protein begins in a tiny cellular factory called a ribosome. There, a protein — a chain of amino acids linked together in a specific configuration — is manufactured on a cellular “assembly line.” Once synthesized the new protein must be shipped to the “address” where it is needed.
One of the major protein transport systems in eukaryotic organisms (organisms whose cells feature a structurally discrete nucleus and other well-developed subcellular compartments) has long been known to principally involve three molecular switches, called GTPases. One of these GTPases, known as SRP 54 — part of a larger complex called the Signal Recognition Particle, composed of six proteins and an RNA molecule — works in concert with GTPases SR alpha and SR beta, the subunits of the two-part SRP receptor.
Schwartz’s structure, formed when the two molecular subunits are bound, establishes that SR beta must be switched on in order to be bound by the other subunit, SR alpha, and form the heterodimer. This new information clears up a widely held misconception in the field of protein transport, according to Schwartz.
Schwartz explains that, without a clear understanding of SR beta’s function, most scientists who study protein transport in cells incorrectly believed that SR alpha and beta were always (and only) in complex — that is, joined as one functional unit — in the cell.
“The function of the two subunits of the SRP receptor was a mystery,” adds Blobel. “Until now, no one really knew what the SR beta switch was good for.”
Neither rain, nor sleet keeps GTPases from appointed rounds
Depending upon their individual functions, freshly manufactured proteins are transported to different destinations as they exit the ribosome. Some stay in the cell; some, such as insulin and other hormones, leave the cell; others must make their way into a cell organelle called the endoplasmic reticulum (ER), where they may end their journey or continue on toward a role in one of the eukaryotic cell’s several other membranes.
Like postal workers at different checkpoints along a delivery route, SRP 54, SR alpha and SR beta work together to ensure delivery to the proper “address” of proteins destined to be exported out of the cell or to do jobs in the cell’s membrane. This address is a channel, located in the ER, through which the proteins travel. Cells to be exported out of the cell are further processed and packed into membrane vesicles that eventually fuse with the cell membrane to release the protein outside the cell. Proteins with jobs to do in the membrane itself enter the channel, and from there are inserted into the membrane — probably through a side opening in the channel, Schwartz and Blobel hypothesize.
Blobel’s Nobel research showed that exported and membrane proteins come out of the ribosome tagged with a characteristic signal peptide (the ZIP Code) comprising the first 20 to 30 amino acids in the chain. As this signal peptide emerges from the ribosome at the head of the nascent chain, SRP 54 recognizes and binds to it. Once this is accomplished, SRP 54, now bound to the ribosome, must somehow find the membrane channel and deposit the nascent protein it has collected. This is done with the aid of the SRP receptor: SR beta anchors the subunit in the membrane; SR alpha signals the channel’s location to SRP.
Alpha-beta soup
Schwartz’s research primarily focuses on SR beta, the anchoring subunit of the SRP receptor. Using X-ray crystallography, he set out to understand how SR beta interacts with SR alpha.
Characterized by two separate domains — SRX and NG — at its opposite ends, SR alpha (signaling subunit of the SRP receptor) serves as the middleman between SRP 54 and SR beta. Along with SR beta, Schwartz crystallized the SRX domain, located at SR alpha’s tail end; this is the domain that binds SR beta. The NG domain, localized at SR alpha’s front end, interacts with the SRP 54-ribosome complex, guiding it to the membrane channel.
“The NG domain is like a flag SR alpha waves around, signaling SRP 54 with the location of the channel,” says Schwartz. It is not yet known whether the SRP receptor first anchors to the membrane, and waves its “flag” from this stationary position; or conversely, waves the flag from a free-floating position in the cell, inducing the SRP 54-ribosome complex to follow it to the membrane channel.
Once the SRP 54-ribosome complex has been signaled by the SRP receptor, with all three molecular switches in the “on” position, a “molecular handshake” occurs between the SRP 54-ribosome complex and the membrane channel. Through a process currently poorly understood, the “ZIP Code” signal peptide is detached from the SRP 54-ribosome complex, and the protein inserted into the channel. Finally, all three GTPases revert to their “off” positions.
What’s next for the researchers? “Now that we understand what SR beta is doing in the cell,” Blobel says, “the question becomes: Why is there regulated assembly of the heterodimeric SRP receptor?” Schwartz adds that he is particularly interested in investigating why the complex, regulated assembly of the heterodimer occurs only in eukaryotes, not in simpler organisms such as bacteria.
This research was funded by the Howard Hughes Medical Institute.