Sperm cells shaped by natural cell suicide mechanism
In male fruit flies, fertility requires it; Trigger for release of “beastly caspases” also identified

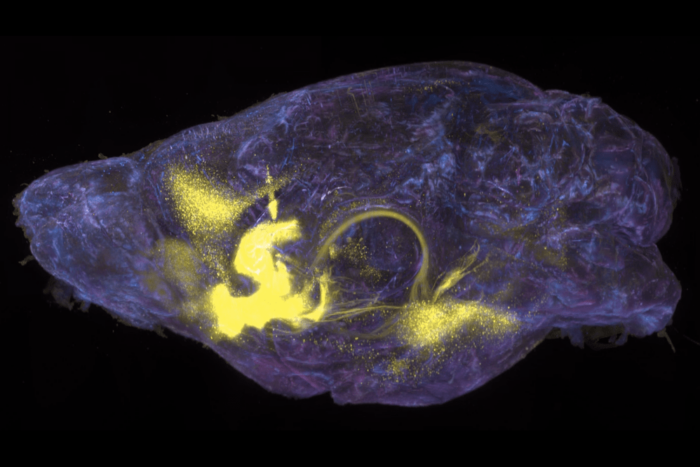
A fly sperm is shown in the process of shedding its cytoplasm. The expelled material is collected in a "waste bag" (stained red) by an apoptosis-like process that requires caspase activity (stained green).
Since discovering that body cells actively commit suicide over 35 years ago, scientists have come to learn that this natural process, called programmed cell death, occurs throughout human tissues, millions of times a day, to eliminate potentially harmful cells, such as those behind cancer.
But what scientists didn’t know until now is that this same mechanism also creates living cells – sperm cells to be exact.
In the May issue of Developmental Cell, researchers at The Rockefeller University report that the final compact shape of a fly sperm cell is in fact the result of cell death. Instead of an entire cell dying as in traditional programmed cell death, the scientists show that just one section of a sperm cell withers away as a regular part of its development.
The findings not only represent the first time that programmed cell death or “apoptosis” has been shown to operate in this partial manner, they also suggest several interesting applications, ranging from insect control to the development of a male birth control pill.
“By understanding how fertile sperm are formed, we might be able to induce infertility in insects and people,” says Hermann Steller, Ph.D., head of the Strang Laboratory of Apoptosis and Cancer Biology at Rockefeller and final author of the new report.
In addition, the study hints at the cause of a common form of male infertility called “droplet sperm.” When the researchers blocked programmed cell death in flies, the insects produced sperm resembling droplet sperm. Though these findings do not immediately translate to treatments for this condition in human males, they may eventually aid in finding one, says Steller.
Finally, the new research also identifies the protein that triggers this apoptosis-like pathway. Previously, it was known that proteins called caspases function as the beastly servants in cell death, tearing up a cell’s innards with murderous intent upon activation. Researchers also assumed that this activation required a mitochondrial protein called cytochrome c, but current data to support this conclusion were not satisfactory. Steller and colleagues now show that a specific form of cytochrome c, called cytochrome c-d, is in fact the culprit.
Other authors of this study include first author Eli Arama, Ph.D., a postdoctoral associate at Rockefeller, and Julie Agapite, a former graduate student at the Massachusetts Institute of Technology.
Streamlined sperm
Sperm cells — in both humans and flies — must go through a series of refinements before they are ready to fertilize an egg. For example, in the human male, a finished sperm cell must be streamlined and compact so that it can make its way through the female’s uterus, and ultimately penetrate her egg. Consequently, during the final stages of this maturation process, called spermatogenesis, a sperm cell loses most of its volume; essentially, its main soupy chamber, the cytoplasm, almost completely breaks away, leaving a highly compressed cell consisting mainly of a nucleus and a long flagellum.
But how sperm cells orchestrate the removal of these “cytoplasmic corpses” was unknown until now.
Cellular sacrifice
An evolutionarily conserved mechanism, programmed cell death operates in a diverse range of organisms, from worms to humans. It takes place during development to sculpt critical organs and tissues, and throughout adulthood to purge the body of unwanted cells. Disruptions to this latter housekeeping activity can lead to a diverse set of diseases in humans: for example, cancer occurs when too little cell death takes place, while Alzheimer’s, Parkinson’s and other disorders arise when too many cells are sacrificed.
Thanks to Steller and other scientists, the nuts and bolts of programmed cell death are now better understood. When it comes time for a cell to kill itself, the body instructs it to do so through a series of molecular messengers that ends with the release of merciless proteins, called caspases, which chew up the insides of cells.
This same pathway holds true for humans and flies, making these insects — which produce some of the longest sperm cells known — excellent models to study cell death. Drosophila melanogaster’s sperm cells are 1.8 millimeters in length, while Drosophila bifurca’s can reach up to 5.8 centimeters. Blue whales produce sperm that are only 0.056 millimeters in length.
Night of the living sperm
Using the fruit fly Drosophila melanogaster as their subject, the Rockefeller researchers decided to test the idea that programmed cell death underlies late events in sperm differentiation. To begin, the researchers looked for caspase activity in developing fly sperm. “Activation of caspases is a key feature of apoptosis, so the presence of active caspases in spermatids indicated the presence of this process,” says Arama. They applied caspase inhibitors to these cells — the equivalent of throwing a wrench in the cell death machinery — and observed that healthy sperm no longer resulted. Instead, the sperm cells still carried excess cytoplasmic baggage, indicating that caspase activity must contribute to the shaping of fertile sperm cells.
Examining mutant, infertile flies next, the scientists discovered more than they hoped for. In addition to identifying an infertile fly with defects in its ability to activate caspases — proof that programmed cell death underlies infertility — they also showed that a mitochondrial protein called cytochrome c-d is responsible for triggering the activation of caspases in fly spermatids. This latter finding represents the first time that cytochrome c was shown to activate caspases in invertebrates, and during normal development in vivo.
“Cytochrome c is a key molecule, which functions in respiration. Drosophila contains two cytochrome c genes and previously people thought cytochrome c-d was the nonfunctional protein of the pair,” says Arama. “But our finding indicates that the two cytochrome c genes fulfill distinct functions in respiration (cytochrome-c-p) and apoptosis (cytochrome-c-d).”
The final question the researchers addressed is how caspases can chew up only part of a sperm cell, leaving the rest intact. Normally, caspases indiscriminately attack everything in their path, but in developing sperm cells, the nucleus and surrounding areas somehow remain untouched. The answer, they discovered, is a protein called dBruce, which is a member of the Inhibitor of Apoptosis (IAP) family of proteins. It turns out that dBruce may neutralize the caspases in those areas that need protection. Precisely how dBruce does this is a question the researchers hope to answer next.
From flies to folks
These experiments, together with a few other key findings, conclusively link programmed cell death to fertility in flies. Whether or not this holds true for humans is currently being addressed in the Steller lab. If there is indeed a human connection, the potential applications are numerous. Along with the possibilities of a male birth control pill, new insect control strategies and a treatment for droplet sperm, there is one immediate ramification.
Presently, researchers are assessing the ability of caspase inhibitors to block cell death in acute alcoholic hepatitis as well as a few other diseases. According to Steller, such treatment should now be monitored for its potential detrimental affects on male fertility. Furthermore, Steller adds that the condition of a patient’s sperm might prove to be a valuable bio-indicator of how well caspase inhibitor treatment is working.
News Releases by this Head of Laboratory
Mice with defective sperm offer clues to infertility in men