World’s first transgenic ants reveal how colonies respond to an alarm
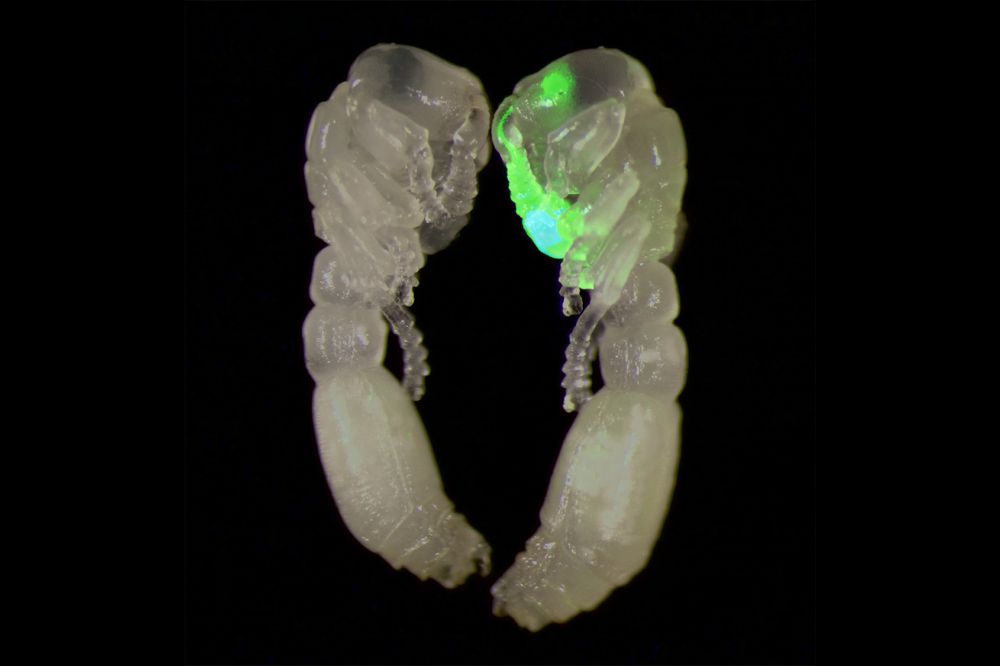
A composite of two clonal raider ant pupa, one transgenic, which expresses the green fluorescent calcium indicator GCaMP in its olfactory sensory neurons, located in the antennae and antennal lobes of the brain. (Taylor Hart)
Ants navigate their richly aromatic world using an array of odor receptors and chemical signals called pheromones. Whether foraging or defending the nest, mating or tending to their young, ants both send and receive chemical signals throughout their lives. The importance of this system is underscored by how well equipped the ant brain is to process the abundance of scents: The olfactory processing center in the ant’s brain has 10 times as many subdivisions as fruit flies do, for example, even though their brains are about the same size.
And yet how the ant olfactory system encodes scent data has remained largely unknown. To whittle away at the mystery, researchers from Rockefeller University have developed the world’s first transgenic ants, which have been bred with olfactory sensory neurons that flash green in response to odorants. They published their results in Cell.
Contrary to previous findings, the study found that only a few specific areas of the olfactory system lit up in response to alarm pheromones, danger signals that elicit panic and nest evacuation. The results raise questions about how sensory information is processed in the ant brain—as well as tantalizing possibilities for revealing what hundreds of other odorant receptors are up to.
“Neurogenetic tools have revolutionized the field of fruit fly neuroscience over the past decades, while social insect neuroscience has essentially been stuck,” says Rockefeller’s Daniel Kronauer, head of the Laboratory of Social Evolution and Behavior. “Our technical breakthroughs now finally allow us to apply these powerful tools in ants to study their social behavior.”
A world of odors
In 1958, E. O. Wilson reported that a secretion from the mandibular gland of harvester ants triggered their nestmates to quicken their pace and take up colony defense behaviors. He called this response “alarm behavior.” Since then, scientists have documented that alarm behavior and many other complex social activities in ant colonies are regulated by a vast array of pheromones.
Ants’ olfactory receptors are located on neurons in their antennae, which send their input to brain centers called the antennal lobes. The antennal lobes are comprised of specialized structures called glomeruli that are essential to scent processing. Some ants have more than 500 glomeruli—a bounty thought to be related to their heightened ability to perceive and discriminate between pheromones. Previous work from Kronauer’s lab has shown that ants whose odorant receptors have been knocked out cannot respond to pheromone signals.
In this study, the researchers created their transgenic subjects by injecting the eggs of clonal raider ants—a queenless species composed entirely of blind female workers—with genetic material encoding the synthetic protein GCaMP, which lights up neon green when calcium levels change during cellular activity.
“Our goal was to get GCaMP expressed only in a single cell type—the olfactory sensory neurons,” says lead author Taylor Hart, a researcher in Daniel Kronauer’s lab.
This was important because the antennal lobe is composed of multiple cell types: sensory neurons, projection neurons that carry sensory data to other parts of the brain, and lateral interneurons that link everything together. “Those other cell types can make signal-to-noise ratio poor, because they can be doing other activities, such as computations, processing information, and modulating signals,” Hart says. All of this can obscure what the olfactory neurons are doing.
Finding the panic button
While successfully breeding a small group of ants with GCaMP expression in the olfactory sensory neurons, the team also developed a sophisticated two-photon calcium imaging technique that allowed them to record neural activity throughout the entire antennal lobes of live ants for the first time.
The researchers decided to focus on alarm pheromones, because they are particularly volatile and elicit strong and robust behavioral responses. They found that adult ants that detected the scents immediately scrambled to gather as many eggs in their mandibles as they could and then made a break for it, fleeing into an adjacent section of the test chamber.
Hart and her team then used their new techniques to monitor GCaMP fluorescence levels in the antennal lobes of 22 transgenic ants as they exposed them to a range of odors, including the alarm pheromones (which smell fruity to the human nose). The flashes clustered in six glomeruli in one region, suggesting that area may act as the brain’s panic button.
“We were expecting that a large portion of the antennal lobe would show some kind of response to these alarm pheromones,” Hart says. “Instead, we saw that the responses were extremely localized. Most of the antennal lobe did not respond at all.”
Hart says the findings reveal details about how the ant brain processes sensory input. Researchers have wondered whether the activity is privatized, with each glomerulus responding only to one or a few specific stimuli, or distributed, with unique combinations of glomeruli activated by a stimulus. A brain with more than 500 glomeruli that operated in a distributed way, with hundreds of sensors firing at once, would need extraordinary computational power when it comes to sensory processing, Hart says.
“Most of the odors we tested activated only a small proportion of the total glomeruli,” she says. “It seems that privatization is the way in the ant antennal lobe.”
Tools for the future
Considering that only six glomeruli responded out of 500, Hart wonders, “What do they need all these different glomeruli for? The fruit fly gets by with just 50.”
It will now be easier to find out why ants have a greater need to differentiate odor stimuli than other insects, Kronauer says—and not only because Hart has since bred hundreds of transgenic ants who differ from their wild counterparts only in their ability to signal in fluorescence, providing a robust pool for future research.
“The tools that Taylor developed open up a really big range of questions that were inaccessible to us until now,” he says. These include associating specific glomeruli with the variety of pheromones ants use for things like raiding, recruitment, and distinguishing between nestmates and outsiders. “There are also interesting developmental questions about how the ant olfactory system gets assembled, because it’s so complex. Larvae also have olfactory sensory neurons, so now we can look at their sensory capabilities.”