For insect cells, like mouse cells, one protein decides between life and death
Cells are given life by mitochondria, an organelle that provides them with all the energy they need. But while mitochondria giveth, they also taketh away — when a cell’s time is up, they release molecules that start a cascade ending in death. At least that’s how it works in humans, mice and other vertebrates. And now, new research from Rockefeller University’s Hermann Steller shows for the first time that the molecules and events that trigger cell death in invertebrates can also start in the mitochondria.

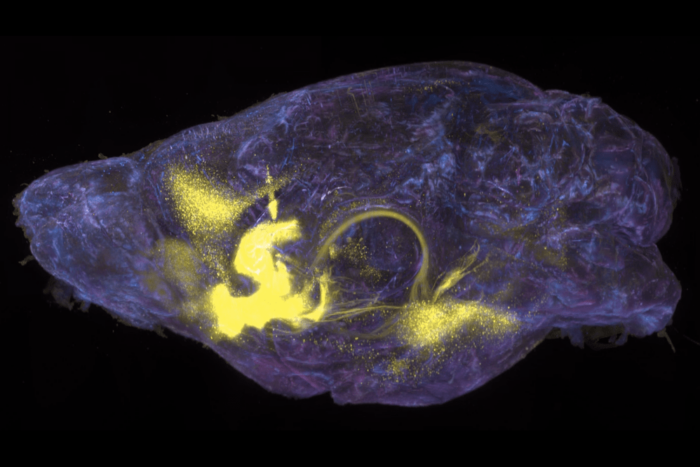
Power cell. Stacks of mitochondria are the power plant that drives a sperm’s ability to swim, but they also control proteins essential for the cell death pathway. During sperm development, mitochondria undergo a striking transformation, fusing to form a spherical mass known as the Nebenkern (highlighted in gold). This image, of mitochondria in fruit fly sperm, was taken with an electron microscope.
Cells die for many reasons: sometimes they initiate death themselves, and sometimes other cells, like immune cells, send the signals. When a vertebrate cell senses a death signal, its mitochondria release a protein called cytochrome c that joins up with two other proteins to create the apoptosome, a multi-protein complex that is the harbinger of death to a cell. It initiates a reaction with enzymes called caspases, dubbed the death cascade. Scientists have known that cytochrome c is important for starting the cascade in vertebrates; what they didn’t know was whether it is necessary in invertebrates, like fruit flies. If it is, flies would provide an amazing genetic tool to study a process that is involved in cancer, autoimmune diseases and neurodegenerative diseases.
The first hint came in 2003 from a study on sperm development in fruit flies, led by Eli Arama, a postdoc in Steller’s lab. For sperm to achieve their streamlined shape, a large amount of excess cytoplasm — the guts of the cell — has to be removed, and Steller’s lab found that this process involves many of the same molecules that are important for cell death. Looking at mutant flies that had trouble producing normal sperm, Steller and colleagues found that cytoplasm removal was defective when one of the flies’ cytochrome c genes, cyt-c-d, was disrupted.
“Research from another lab saw similar results, but they also suggested that these mutants might have other genes affected, not just cyt-c-d,” says Steller. “So we went back to rigorously address this issue, through genetics and rescue experiments, to unequivocally establish a role for cytochrome c in the activation of caspases.”
In the mutant flies, the scientists, again led by Arama, were able to rescue their defective sperm by giving the flies another copy of the cyt-c-d gene — if other genes were also affected, this strategy would not have worked. Also, they found another cyt-c-d mutant, this time with only one amino acid changed in the protein, which also caused flies to produce defective sperm. Together, these results solidified what they had reported in the first paper: Cyt-c-d is crucial for caspase activation during sperm development. The next question was to ask if cyt-c-d was playing a role in apoptosome formation in the sperm.
Steller’s lab went on to look at the fly equivalents of the other two proteins that make up the apoptosome in vertebrates, Apaf-1 and caspase 9. They found that, just as with cyt-c-d, both are required for normal sperm development. If either one is removed, the same defects that occur when cyt-c-d is mutated come back. These data provide the first evidence of an apoptosome in invertebrates, showing that the death cascade in mammals and flies is incredibly similar.
“There may be other pathways involved in caspase activation — it is a more complex process than anyone thought,” Steller says. “But this study demonstrates that late spermatogenesis in the fruit fly is a powerful system that can be used to identify novel pathways for caspase regulation.”
In addition to defects in sperm development, which are a major cause of infertility in men, problems with the regulation of the cell death pathway play a role in cancer, autoimmune diseases like lupus, and neurodegenerative diseases such as Alzheimer’s. Using the genetic tools available in the fruit fly, more information on how the death pathway and the apoptosome function may shed new light on these conditions.