A missing antibody molecule may indicate when dengue will become deadly
A first encounter with the dengue virus typically causes very mild symptoms; however, a subsequent infection is a different story. For a small proportion of people who are reinfected, the virus can cause severe symptomatic disease, which is often life-threatening.
“The main hypothesis for some time has been that antibodies generated the first time around, instead of providing protection against disease, can actually exacerbate it,” says Stylianos Bournazos, research assistant professor at Rockefeller. “But even in secondary infection, we see a wide range of symptoms—so the presence of antibodies alone cannot explain why only some cases turn deadly.”
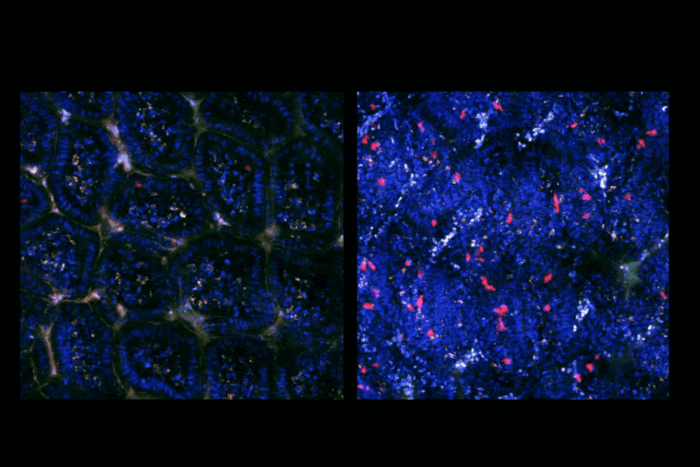
Now new findings published in Science by the lab of Jeffrey V. Ravetch in collaboration with the Pasteur Institute in Cambodia suggest that the susceptibility and severity of dengue disease comes down to a particular type of antibody that is missing a specific sugar, fucose, on its stem. This impacts the antibody’s so-called Fc region, which is responsible for binding and passing instructions along to other immune cells.
Previously, researchers in the Ravetch lab found that patients with severe dengue disease have unusually high levels of these fucose-less antibodies. However, it was not clear whether the absence of fucose was the result of severe disease or its cause.
By analyzing samples from a variety of dengue patients early in the onset of their disease, the team found that those who eventually developed the most severe disease also had significantly higher levels of fucose-deficient antibodies at the time of hospital admission. As a result of this change to their structure, the antibodies bind too strongly to white blood cells, increasing inflammation and leading to the destruction of platelets crucial for blood clotting. The result is hemorrhagic fever and shock syndrome often seen in severe dengue disease.
The findings suggest that the fucose status of antibodies represents a robust prognostic tool, says Ravetch, Theresa and Eugene M. Lang Professor and head of the Leonard Wagner Laboratory of Molecular Genetics and Immunology. “It can help us identify patients at risk of severe illness so they can receive appropriate medical care early on.”