Bacteria in the gut have a direct line to the brain
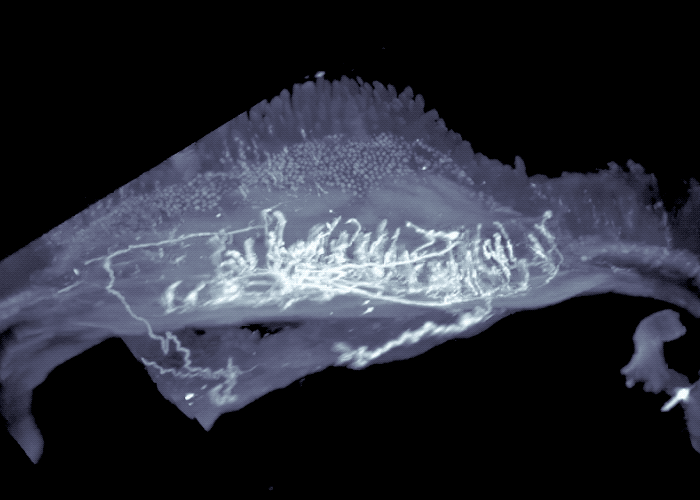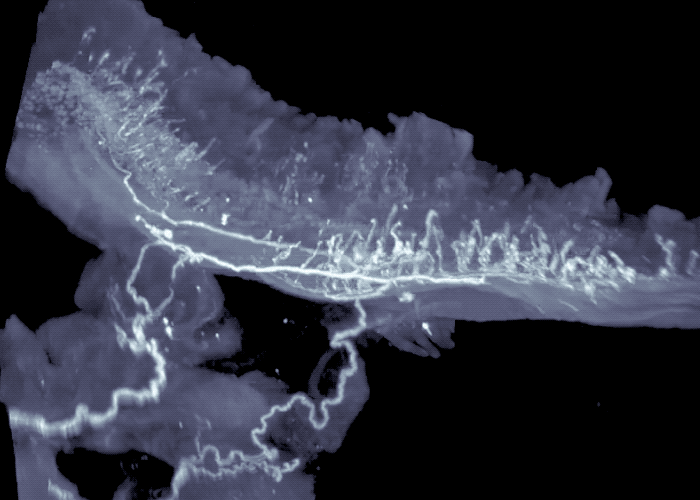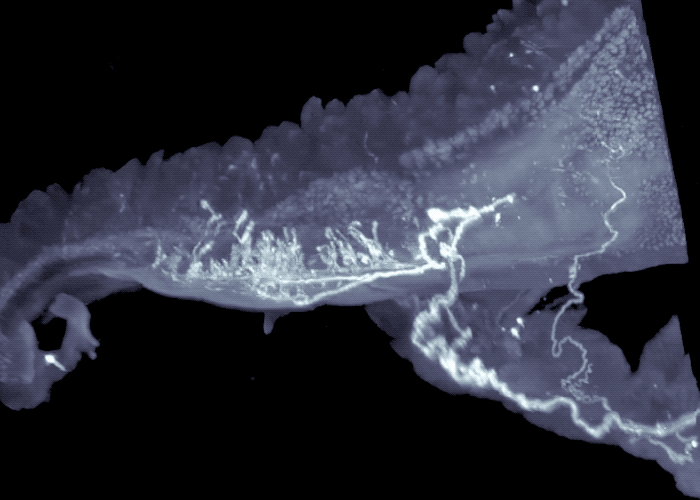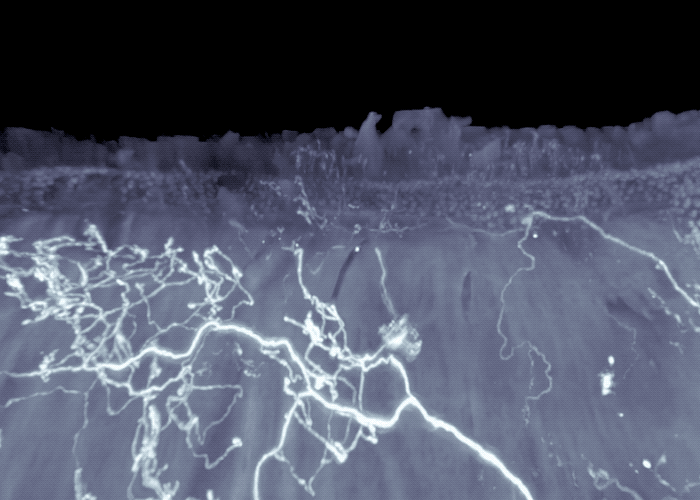
Three-dimensional view of mouse intestine shows sensory neurons in areas exposed to high-levels of microbial compounds.
With its 100 million neurons, the gut has earned a reputation as the body’s “second brain”—corresponding with the real brain to manage things like intestinal muscle activity and enzyme secretions. A growing community of scientists are now seeking to understand how gut neurons interact with their brain counterparts, and how failures in this process may lead to disease.
Now, new research shows that gut bacteria play a direct role in these neuronal communications, determining the pace of intestinal motility. The research, conducted in mice and published in Nature, suggests a remarkable degree of communication between our nervous system and the microbiota. It may also have implications for treating gastrointestinal conditions.
“We describe how microbes can regulate a neuronal circuit that starts in the gut, goes to the brain, and comes back to the gut,” says Rockefeller’s Daniel Mucida, associate professor and head of the Laboratory of Mucosal Immunology. “Some of the neurons within this circuit are associated with irritable bowel syndrome, so it is possible that dysregulation of this circuit predisposes to IBS.”
The work was led by Paul A. Muller, a former graduate student in the Mucida lab.
How microbes control motility
To understand how the central nervous system senses microbes within the intestines, Mucida and his colleagues analyzed gut-connected neurons in mice that lacked microbes entirely, so-called germ-free mice that are raised from birth in an isolated environment, and given only food and water that has been thoroughly sterilized. They found that some gut-connected neurons are more active in the germ-free mice than in controls and express high levels of a gene called cFos, which is a marker for neuronal activity.
This increase in neuronal activity, in turn, causes food to move more slowly than usual through the digestive tract of the mice. When the researchers treated the germ-free mice with a drug that silences these gut neurons, they saw intestinal motility speed up.
It’s unclear how the neurons sense the presence of gut microbes, but Mucida and his colleagues found hints that the key may be a set of compounds known as short-chain fatty acids, which are made by gut bacteria. They found that lower levels of these fatty acids within the guts of mice were associated with greater activity of the gut-connected neurons. And when they boosted the animal’s gut levels of these compounds, the activity of their gut neurons decreased. Other microbial compounds and gut hormones that change with the microbiota were also found to regulate neuronal activity, suggesting additional players in this circuit.
Neurons in control
Further experiments revealed a conundrum, however. The scientists saw that the particular type of gut-connected neurons activated by the absence of microbes did not extend to the exposed surface of the intestines, suggesting that they cannot sense the fatty acid levels directly.
So Mucida and his colleagues decided to trace the circuit backwards and found a set of brainstem neurons that show increased activity in the germ-free mice. When the researchers manipulated control mice to specifically activate these same neurons, they saw an increase in the activity of the gut neurons and a decrease in intestinal motility.
The researchers continued to work backwards, next focusing their attention on the sensory neurons that send signals from the intestines to the brainstem. Their experiments revealed these sensory neurons extended to the interface of areas of the intestine that are exposed to high-levels of microbial compounds, including fatty acids. They turned off these neurons, to mimic what happens in germ-free mice that lack the fatty acids, or associated gut signals, and observed activated neurons in the brainstem, as well as activation of the gut neurons that control intestinal motility.
“We traced the whole loop and saw that neurons outside the intestines can be controlled by what happens inside the intestines,” Mucida says. “It is plausible that the circuit identified here could be involved in additional gut-brain bidirectional interactions, which could influence several intestinal as well as neurological diseases, including IBS and even behavioral abnormalities.”



