Structural images shed new light on a cancer-linked potassium channel
Most cells in the body carry on their surface tiny pores through which potassium ions travel. In controlling the flow of these positively charged ions, the channel helps the cell maintain its electrical balance.
One particular type of potassium channel, called Eag1, has been found in a number of cell types: in the neurons of the brain, in embryonic cells that generate muscle fiber, and in some tumor cells, where it’s thought to have a cancer-promoting effect. But it’s not yet clear how Eag1 differs from other potassium channels, or exactly how it works.
A duo of researchers at The Rockefeller University has taken an early step toward an answer. Using Rockefeller’s new facility for cryo-electron microscopy, an advanced imaging technique in which samples are frozen then bombarded with electrons, they determined the structure of Eag1. Their results, which appear August 11 in Science, have provided the first 3-D structure of a molecule to be published from Rockefeller’s facility.
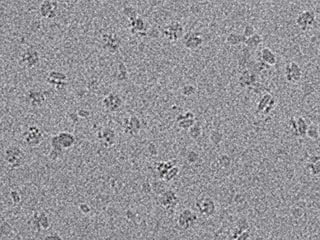
New views: Samples of the channel were frozen, then bombarded with electrons to produce the raw images shown above. This work was done in Rockefeller’s new cryo-electron microscopy facility.
Like some other potassium channels, Eag1 opens when it senses a change in electrical potential, as happens when neurons send signals. In the video above, the part of the channel that most interested the researchers—the section that spans the cell membrane—appears in yellow and green.
It includes the sensors responsible for detecting electrical changes (yellow), and the segments that form the pore through which potassium passes (green). The rest of the channel is located inside the cell. The researchers also determined the structure of another molecule called calmodulin (purple), which binds to Eag1 and holds it in a closed position.
“Within the structure, we see some important differences between Eag1 and other potassium channels in the section that spans the cellular membrane,” says first author Jonathan Whicher, a postdoc in Roderick MacKinnon’s lab. “This gives us a better idea of how the channel’s components work on a molecular level, and its role within a cell, either a normal one or a cancerous one.”
This research is an early step toward finding molecules that could inhibit or control the channel. These, in turn, could provide valuable tools for further exploring the role of Eag1 in cancer, or for developing new therapeutics.
The study also begins to fill an important gap in understanding the function of potassium channels, which are the primary focus of MacKinnon’s Laboratory of Molecular Neurobiology and Biophysics. In 2003, MacKinnon, who is also the John D. Rockefeller Jr. Professor and a Howard Hughes Medical Institute investigator, won the Nobel Prize for capturing the 3-D structure of a potassium channel for the first time.
This work was supported by the National Institutes of Health (grant GM43949) and the Damon Runyon Cancer Research Foundation (DRG-2212-15).
 Science 353, 664–669 Science 353, 664–669Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism Jonathan R. Whicher and Roderick MacKinnon |