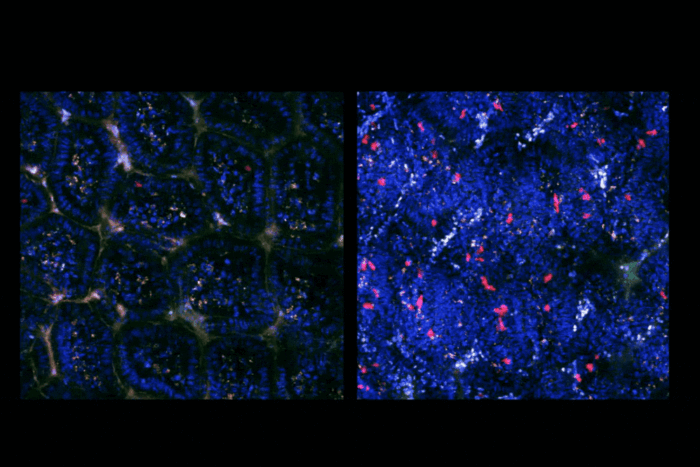
Studying the cleanup crew of the genome to illuminate a rare disease

When Agata Smogorzewska told her father in 2009 that she was starting her own lab called the Laboratory of Genome Maintenance, he balked at the name.
“It sounds like you’re going to have a cleaning service,” he protested.
Smogorzewska, who had accepted a junior faculty position at Rockefeller, was used to her family of scientists weighing in on her choices—and then choosing her own path anyway. Her father is a nephrologist, her mother an immunologist, and her aunt a polymer chemist. Her grandparents had been chemists, too. She’d learned the importance of precision early: As a child she’d spent time in her grandmother’s university lab in Warsaw, Poland, tasked with carefully monitoring the drops of liquid passing through an enormous glass distillation apparatus mounted on the wall.
Notwithstanding her father’s complaint, Smogorzewska knew that the name she had chosen was perfect. The Laboratory of Genome Maintenance investigates the clean-up crew of the genome—the handful of DNA repair mechanisms that attempt to correct problems, errors, and breakdowns.
Our genomes are constantly accumulating damage from both external sources like sunlight and toxins and internally during DNA replication. Each one of us is, genetically speaking, a bit like a ramshackle house where a team of molecular handymen are working 24/7 to fix creaky beams, clogged pipes, and faulty wiring. If left uncorrected, these mistakes can compound, making the genome unstable and potentially leading to cancers and other disorders.
Such is the case in people with Fanconi anemia, a rare disease caused by inherited mutations in some of the genes responsible for the genome’s maintenance. Smogorzewska’s lab uses the disease as an entry point to understand the mechanisms of DNA repair, and has discovered specific mutations that can lead to bone marrow failure, skeletal deformations, and a high risk of cancer. Over the years, her research has turned Fanconi anemia into a model syndrome for understanding genome maintenance itself.
How, but not why
At Rockefeller, Smogorzewska’s aim is to make important discoveries about the fundamental mechanisms of life—an idea that has appealed to her since she was in high school biology class in Warsaw in the early 1990s, peering at cells through a microscope. The textbook on her desk explained how some fundamental cellular process occurred, but not why. “I felt that there had to be something more to it,” she recalls. “The microscopic level was more interesting to me than the macroscopic level.”
Living independently in the Polish capital—her parents had permanently relocated to Los Angeles for work—Smogorzewska was expected to apply to medical school. But she wanted to join her parents in California to study molecular biology, whose unknowns intrigued her. “I thought it would be more fun than staying in Poland and training to become just a physician,” she says.
So on the day of the med school entrance exam, surrounded by nervous students dressed in formal white and blue, she intentionally flunked the test, making just enough errors to tank it without raising suspicions.
“It was the only way my dad would say, ‘Ok, fine, come to the States,’” she says.
After completing her degree in molecular biology and biochemistry at the University of Southern California, she looked to New York City, whose rich cultural offerings reminded her of Warsaw, where she’d grown up going to the philharmonic and the opera. “It helped that I didn’t like California very much,” she admits. “This sunny disposition does not exactly fit me.”
New York, New York
Forging her own path once again, she soon secured a spot in the Tri-Institutional M.D.-Ph.D. Program that Rockefeller runs together with its neighboring institutions, Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center. She interviewed with pioneering molecular geneticist Norton Zinder, who seemed uninterested in their conversation, feet up on his desk, newspaper spread between his hands—until she disagreed with him about the F plasmid, a type of bacterial DNA that can transfer genes from one cell to another. “He perked up when I started arguing with him,” she laughs.
Her PhD work in Titia de Lange’s lab at Rockefeller set her on the path to study DNA repair. She found that telomeres—protective caps on the ends of chromosomes that prevent them from tangling, fraying, or connecting—sometimes fuse chromosomes together, potentially leading to lethal results. Later, as a postdoc at Harvard Medical School in the lab of molecular biologist Stephen Elledge, she was able to shed light on the mechanism that normally prevents such mistakes, in part by demonstrating that the gene FANCI is involved in DNA interstrand crosslink repair, a process that severs covalent bonds that can form between the strands of DNA during replication.
Soon after, she went to a conference on Fanconi anemia where, for the first time, she met with people suffering from the disease. “The patients were really interesting—much more complex than the textbook descriptions of their symptoms, such as having an inability to make blood components or having arm defects. They were also all very unique, and many were adults, not children, which I did not expect,” she says. “The disease had aspects that people didn’t understand at all, including why they were predisposed to specific cancers. It seemed like a really good time for a physician-scientist to work on it.”
Those patients, it turned out, were waiting for her at Rockefeller.
From registry to discovery
DNA interstrand crosslink repair is mediated by the Fanconi pathway, a genome-integrity mechanism with 22 genes identified so far. If any of these genes are faulty, the process fails, leading to extreme genome instability. People with Fanconi anemia have a very high risk of developing squamous cell carcinomas. They’re 500 times more likely to get highly metastatic head and neck cancers, and thousands of times more likely to get vulvar cancer.
A decades-old patient registry has been central to Smogorzewska’s research. Established in 1982 by Rockefeller geneticist Arlene Auerbach, the International Fanconi Anemia Registry (IFAR) now has more than 1200 families representing about 4500 people—a rich resource for such a rare disease. Auerbach, who created a diagnostic test for the disease and identified the gene responsible for 65 percent of cases, retired from Rockefeller the year Smogorzewska arrived, making for an auspiciously smooth inheritance.
“I inherited a ton of cell lines, a ton of materials,” she says. “I knew coming in that I would be able to identify new genes. And in time, we did.”
When Smogorzewska and her colleagues have a scientific question, they go to the registry and pull out the patients who might help them answer it. It’s through the registry that her lab, for example, identified mutated SLX4, RAD51, and UBE2T genes in Fanconi anemia patients and discovered that DNA replication errors in the tissues of their tumors are similar to those of people who smoke, providing a unique clue to how smoking-related cancers are triggered in the general population.
She’s using next-generation sequencing to identify gene variants, with the ultimate goal of pinpointing treatment targets. These are especially important for people with Fanconi anemia, who can develop highly aggressive sporadic head and neck cancers that can’t be treated with chemotherapy because of their inability to repair DNA. These cancers are also common in people without Fanconi anemia, so “if you study this disease as a model and identify treatments, that can help patients in the general population,” she says.
Smogorzewska’s lab is also seeking to better understand other members of the genome’s clean-up crew, because a range of disparate conditions share one thing in common: a tendency to have a shaky genetic foundation. Some of the mechanisms they study, for example, help DNA replication stay on track. Errors and failures in the code-copying process cause many diseases, especially in areas of the genome that have clusters of highly repetitive code, which can bind together to form blockages that stop the replisome, or DNA replication machinery, from working. Proteins traveling with the replisome can remove these blockages and restart the machinery once the path is cleared.
“But there are lots of ways of doing it, and we don’t know all of them still,” she says.
It’s those kinds of molecular unknowns about the genetics of disease that will continue to inspire Smogorzewska’s research—and perhaps provide some whys for the textbooks of the future.