Faculty members Kivanç Birsoy and Jun Cao receive promotions
The Committee on Scientific Affairs of the University’s Board of Trustees has awarded promotions to two faculty members: Kivanç Birsoy has been promoted to professor with tenure and Junyue Cao has been promoted to associate professor. In addition, Birsoy has been named the Joseph L. Goldstein Professor. Birsoy is the head of the Laboratory of Metabolic Regulation and Genetics. Cao is the Fisher Center Foundation Associate Professor, and head of the Laboratory of Single-Cell Genomics and Population Dynamics. Both promotions are effective January 1, 2026.
Birsoy is a product of Rockefeller’s graduate program, completing his Ph.D. thesis in Jeffrey Friedman’s lab in 2010 before doing postdoctoral research at the Whitehead Institute at MIT. He returned to Rockefeller in 2016 as assistant professor and was promoted to associate professor in 2023. He has been recognized with numerous awards, including the Vilcek Prize, the Blavatnik National Award in Life Science (finalist), the Sable Award in Metabolic Physiology, and the American Society of Cell Biology Innovation in Research Award.
 The Birsoy lab studies how metabolic pathways regulate biological processes and contribute to diseases including cancer, mitochondrial disorders, and inborn errors of metabolism. Since joining Rockefeller, Birsoy has made many contributions to our understanding of metabolism. He solved the long-standing question of how glutathione—the major antioxidant in cells–gets into mitochondria, where it is essential for neutralizing the high levels of reactive oxygen species produced there. He identified the essential mitochondrial glutathione importer, SLC25A39, and went on to characterize the autoregulatory mechanism by which levels of glutathione in mitochondria regulate the proteolytic degradation of the transporter. He demonstrated the importance of this process by showing SLC25A39 is essential for red blood cell development and that mice that are genetically modified to lack it do not survive. He also showed that high mitochondrial glutathione is essential for breast cancer cells to successfully establish metastatic colonies, reflecting the harsh environment experienced by cancer cells as they invade tissues.
The Birsoy lab studies how metabolic pathways regulate biological processes and contribute to diseases including cancer, mitochondrial disorders, and inborn errors of metabolism. Since joining Rockefeller, Birsoy has made many contributions to our understanding of metabolism. He solved the long-standing question of how glutathione—the major antioxidant in cells–gets into mitochondria, where it is essential for neutralizing the high levels of reactive oxygen species produced there. He identified the essential mitochondrial glutathione importer, SLC25A39, and went on to characterize the autoregulatory mechanism by which levels of glutathione in mitochondria regulate the proteolytic degradation of the transporter. He demonstrated the importance of this process by showing SLC25A39 is essential for red blood cell development and that mice that are genetically modified to lack it do not survive. He also showed that high mitochondrial glutathione is essential for breast cancer cells to successfully establish metastatic colonies, reflecting the harsh environment experienced by cancer cells as they invade tissues.
Birsoy has also identified other mechanisms that allow cancers to survive in harsh environments or prevent attack by the immune system, including mechanisms that are specific to tumor types. In one of these, he showed that pancreatic cancer cells can prevent immune cell attack by producing high levels of glycosphingolipids. Inhibition of their synthesis allows the immune system to kill the cancer cells, suggesting a potential benefit of combining a traditional pharmaceuticals with an immune checkpoint inhibitor. He also showed that certain lymphomas lose an enzyme in the cholesterol biosynthetic pathway that cause them to produce high levels of squalene, which protects the cancer cells from lipid peroxidation and cell death. This work suggests that because these cells can no longer make cholesterol, inhibiting its uptake via the LDL receptor would be expected to have a therapeutic benefit.
 Cao did his Ph.D. at the University of Washington, and stayed for another year before being recruited to Rockefeller in 2020. His graduate research revolutionized the growing field of single cell RNA sequencing by developing new methods, including one known as sci-RNA-seq, that use combinatorial indexing to eliminated the need to isolate and extract RNA from individual cells, providing higher throughput at a dramatically lower cost. He used this method to track the developmental trajectory of cells as they differentiate in distinct lineages throughout development. This work won him the Science Magazine Grand Prize for Young Scientists.
Cao did his Ph.D. at the University of Washington, and stayed for another year before being recruited to Rockefeller in 2020. His graduate research revolutionized the growing field of single cell RNA sequencing by developing new methods, including one known as sci-RNA-seq, that use combinatorial indexing to eliminated the need to isolate and extract RNA from individual cells, providing higher throughput at a dramatically lower cost. He used this method to track the developmental trajectory of cells as they differentiate in distinct lineages throughout development. This work won him the Science Magazine Grand Prize for Young Scientists.
Cao has continued to develop innovative methods for genomic analysis at Rockefeller. These include the simultaneous characterization of RNA transcripts and open chromatin domains in chromosomes (genomic sites that regulate RNA transcription); the ability to distinguish RNAs that are newly made from those that are older; and the identification of cells that are replicating their DNA, marking proliferating cells.
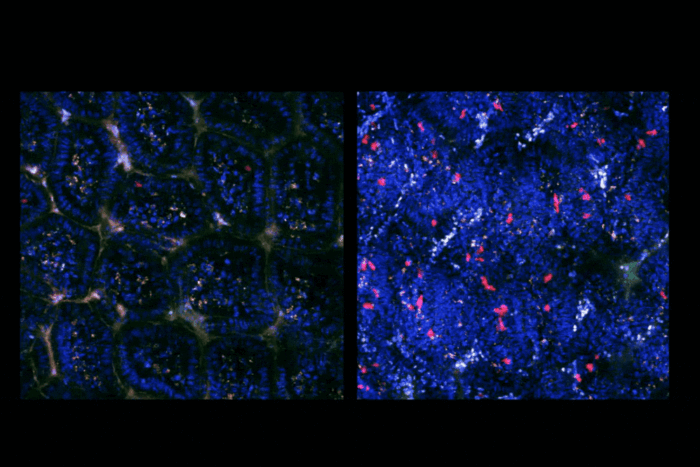
Cao came to Rockefeller with an ambitious goal: to understand the mechanisms of aging and its consequences, such as neurodegeneration. He sought to begin by determining how the numbers of all the cell types in the body vary over an organism’s lifespan, and characterized over 20 million cells from 14 organs, in male and female mice, of three different mouse strains, at five different ages. The results suggest that aging does not entail simply the gradual loss of cell numbers over time, but instead showed that populations of some cell types declined in two distinct waves in mid-life, while those of others, particularly cells of the immune system, expand in two waves at older ages. Cao also showed that declining cell types further reduce their proliferation over time, while expanding populations continue to increase.
In separate studies, he showed that cell division and differentiation in the human and mouse brains show an aging-related defect in the differentiation in oligodendrocytes, and that a classical aging-related intervention, dietary restriction, can partly restore neurogenesis and ameliorate oligodendrocyte defects in aging mice.