Equipment
Light microscopy
Microscopes (DWB room 201, 202 and 203)
Current equipment in the light microscope facility includes:
1. Wide-field fluorescence/brightfield/DIC microscope (Zeiss) – “Edwina”
- Axioplan 2 imaging upright microscope
- Wide range of objectives
- Filter sets for DAPI, CFP, GFP, AF 488, YFP, Texas Red, Cy5
- Brightfield and Differential Interference Contrast (DIC)
- Spot Insight QE color digital camera for brightfield (and fluorescence) imaging or Hamamatsu Orca ER B/W digital camera for sensitive fluorescence imaging
- Motorized Z-drive
- MetaVue acquisition software (Molecular Devices)
2. Inverted microscope for long-term time-lapse, fluorescent and phase contrast imaging of cells or embryos (Olympus) – “Wendolene”
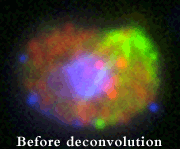

- Olympus IX71 inverted microscope with phase contrast optics
- 10x, 20x, 40x, 60x and 100x objective lenses, some suitable for use with plastic as well as glass dishes/plates
- Filter sets for DAPI, CFP, GFP, YFP, RFP and Cy5
- Hamamatsu Orca ER B/W digital camera and QI Click color digital camera
- Prior XYZ piezo stage (200 microns z-travel) for multiple point visiting during timelapse acquisitions
- MetaMorph acquisition software (includes MDA)
- Solent Scientific environmental chamber for temperature regulation
3. DeltaVision Image Restoration Microscope (Applied Precision/GE/Leica) – “Wallace”
- Inverted Olympus IX-70 microscope
- 40x, 60x and 100x oil objectives, 60x water objective, 60x and 100x Silicone oil objectives, 20x dry objective, plus 1.5x optovar
- Filter sets for DAPI, CFP, GFP/FITC, YFP, Rhodamine, AlexaFluor 594/mCherry and Cy5
- DIC
- Separate excitation and emission filter wheels (necessary for FRET)
- pco.edge sCMOS camera
- Extremely precise stage allowing multiple point visiting and image stitching
- Insight SSI 7 color solid state illumination system
- Quantitative
- Koehler and critical illumination
- Deconvolution using measured PSFs
- Warner Instruments stagetop imaging chambers
4. 2nd DeltaVision Image Restoration Microscope (Applied Precision/GE/Leica) – “Totty”
- All the features of the above system plus a FRAP/photoactivation module with 405 and 488 lasers
- “Ultimate Focus” – hardware-based autofocus system for long-term timelapse imaging without focal drift
- Weatherstation environmental chamber – can be set at temperatures from ambient temperature to 37°C
5. CellDiscoverer7 (CD7) automated widefield high-throughput system (Zeiss) – “Admiral Collingwood”
- 37 degree incubator microscope with 5% CO2 for multi-day imaging of fixed or living samples
- Autodetection of a variety of chamber/plate formats for easy multi-point visiting and tiling
- Hardware- and software-based autofocus and autocorrection of objectives
- Multi-color, multi-position, multi-z imaging
- Filter cubes for DAPI, CFP, GFP, YFP, Rhodamine/DsRed, mCherry, Cy5
- 5x/0.35, 20x/0.70, 20x/0.95 dry objectives and 50x/1.2 water objective with 0.5x and 2x optical magnification changers
- Controlled by ZenBlue acquisition and analysis software
6. Inverted FluoView FV4000 laser scanning confocal microscope (Evident) – “Norbot”
- Inverted Evident IX-83 microscope with Zero Drift Compensation (ZDC830)
- 405, 445, 488, 514, 561, 594, 640, 685, 730 and 785 nm laser lines (solid state)
- Six next-generation ultra-sensitive, quantitative and high dynamic range SilVIR spectral detectors (combination of broadband and red-shifted)
- TruSpectral high sensitivity multichannel imaging from 400 through 900 nm
- Evident CellSens FV Software including FRAP and FRET
- Wide range of dry, oil and silicone oil objectives, including the highly corrected X-line series
- XY scanning motorized stage for advanced tiling capabilities
- Galvo scanner for best signal:noise and resonant scanner for highest-speed applications
- Inbuilt Microscope Performance Monitor for assessing laser power stability and microscope performance over time
- Tokai Hit stage-top incubator for heat and CO2 control
7. Inverted LSM 980 laser scanning confocal microscope (Zeiss) – “Molly”
- Inverted Zeiss Axio Observer Z1/7 microscope with Definite Focus
- 405, 445, 488, 514, 561, 594, 639 and 730 laser lines
- 34 spectral detection channels via GaAsP PMT detector. two multialkali PMT, one NIR GaAS PMTs, one NIR GaAsP PMT and one Airyscan detector
- Monochrome camera Axiocan705
- LED fluorescence illumination (Collibri5)
- Zen Blue 3.5 Software with Fluorescence Correlation Spectroscopy (FCS), FRET and FRAP modules
- Wide range of oil and water, glycerol immersion objectives
- XY scanning motorized stage for advanced tiling capabilities
- Stage-top incubator for heat and CO2 control
8. Inverted LSM 880 Airyscan NLO laser scanning confocal and multiphoton microscope (Zeiss) – “Rocky II”
- Inverted Zeiss Axio Observer Z1 microscope
- 405 nm, 458, 488, 514, 561 and 633 laser lines
- Chameleon Ultra II NIR laser fully tunable between 690 and 1040 nm
- Airyscan detector for enhanced resolution and sensitivity
- GaAsP detector for sensitive confocal imaging
- BiG.2 NDD detector for multiphoton imaging
- X-Y motorized stage for tiling and multiple point visiting
- Wide range of oil, glycerol and water immersion objectives
- Simultaneous 3-channel fluorescence and DIC imaging
- FRAP and FRET modules
- Full environmental chamber for heating/CO2
9. RS-G4 resonant scanning confocal system (Caliber ID) – “Cutlass Liz“
- High-speed strip-mosaic confocal imaging and large image stitching, up to 4 slides at a time
- Flexible scan head
- 405, 488, 568, 638 and 785 nm laser lines
- A wide variety of compatible objectives including Olympus XApo 20x/0.8 dry (for slides) and Nikon CFI90 Apo 20x/1.00 glycerol with 8.2 mm WD (for cleared tissue)
- Cleared tissue kit includes chambers of different heights to accommodate thick, cleared specimens (up to around 8 mm)
10. Facility Line STED/confocal system (Abberior) – “Pirate King“
- New “Facility Line” model of Stimulated Emission Depletion super-resolution system for 2D and 3D STED
- 775 nm pulsed depletion laser
- Pulsed 405, 485, 561 and 640 nm excitation lasers permit time gating
- Auto-alignment
- Adaptive STED illumination package (DyMIN/RESCue) for live imaging
- SLM (Spatial Light Modulator) for dynamic beam shaping and aberration control
- Easy version of Imspector software tailored for core facilities or Expert Line software for advanced applications
- Full confocal system with spectral detection based on ultra sensitive single-photon-counting APD detectors
- Transmitted light detector (currently only available in Expert Line mode)
- Olympus IX83 stand with ZDC-12 hardware autofocus
- 20x/0.80 dry, 40x/1.4 oil, 60x/1.30 Silicone oil, 100x/1.35 Silicone oil and 100x/1.40 oil objectives
11. InstantSIM (iSIM) real-time super-resolution system (VisiTech/BioVision/Leica/Mizar) – “Scopey McScopeface“
- VisiTech iSIM head with Variable Pinhole and Ingwaz
- Leica DMi8 inverted microscope stand with hardware-based autofocus and DIC capabilities
- 20x/0.75 dry, 25x/0.95 water, 63x/1.30 glycerol, 93x/1.30 glycerol (with motorized correction collar) and 100x/1.47 oil objectives
- Two Orca Fusion sCMOS cameras for simultaneous 2-channel acquisition or rapid switching multichannel acquisition
- Mizar TILT light sheet module for rapid and gentle imaging mode
- VisiView acquisition software and VisiFRAP module for photobleaching, photoactivation and photoablation
- Microvolution deconvolution software to achieve full resolution enhancement
- Okolabs chamber for environmental control, including cooling option
12. OMX Blaze 3D-SIM super-resolution microscope (Applied Precision/GE/Leica) – “Reverend Hedges”
- 405, 445, 488, 514, 568 lasers for 3D-SIM super-resolution imaging
- 6-line SSI module for ultra-rapid conventional imaging
- 100x/1.40 UPLSAPO oil or 100x/1.35 Silicone oil objective (Olympus)
- Three Evolve EMCCD cameras (Photometrics) for simultaneous or sequential acquisition
- Heating chamber for live cell imaging
13. FV1000MPE upright multiphoton system (Olympus) – “Philip”
- Coherent Chameleon Vision II IR laser with integrated dispersion compensation and a wide tuning range of 680 to 1080 nm
- Olympus BX61 upright microscope
- 473 nm visible laser for imaging or stimulation
- 405 nm laser for visible stimulation
- Range of objectives including 25x/1.05 NA Plan objective optimized for infrared wavelength transmission and deep-tissue imaging
- Wide range of filter sets
- Prior motorized stage for multiple point visiting
- Inhalation anesthesia system (VetEquip) for long term live imaging
- Biostage 600 warming plate (20-20 Technology)
- Olympus DP-20 camera for accurate repetitions of live sample positioning
14. Spinning disk confocal microscope for live cell imaging (Zeiss/Yokagawa/Spectral Applied) – “Bunty”
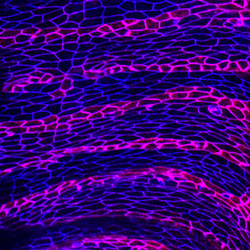
Benjamin Boettner and Ulrike Gaul (Rockefeller University)
- Inverted Zeiss Axio Observer Z1 microscope
- Wide range of oil, glycerol and water immersion objectives
- Yokagawa CSU-10 spinning disk confocal head with Spectral Applied Borealis upgrade
- Solid-state 443, 491, 523, 561 and 644 lasers for excitation (Spectral Applied)
- Photonics Instruments Digital Mosaic system with 405 nm laser for performing ROI FRAP and photoactivation;
- Choice of Hamamatsu Flash 4.0 sCMOS camera or Andor iXon 512×512 EMCCD camera for high resolution or high sensitivity/speed imaging
- Prior XYZ piezo stage for multiple point visiting during timelapse acquisitions
- Image acquisition performed with MetaMorph software
- Eppendorf microinjection system
- Solent Scientific environmental chamber for control of temperature and CO2
15. CellVoyager (Yokagawa/Olympus) – “HMSBeagle“
- Spinning disk confocal system
- Two disks with different pinhole sizes or widefield illumination
- 445, 488 and 561 nm lasers
- Hamamatsu 512 x 512 EMCCD camera
- 10x and 20x dry objectives, 30x and 60x silicone oil objectives
- Incubation temperature from 18 degC to 40 degC, CO2 optional
16. Ring-TIRF system (Nikon) with iLAS2 for FRAP/photostimulation – “Piella”
- Nikon Ti2E inverted microscope with Perfect Focus mechanism
- Ring-TIRF, spot-TIRF, HILO and widefield illumination options
- 405, 488, 561 and 640 nm laser lines for TIRF
- ILAS2 for FRAP or Photostimulation
- CoolLED pE4000 for widefield illumination
- Piezo x,y,z stage
- Andor DU-897 EMCCD camera
- Orca-FusionBT liquid cooled sCMOS camera
- Tokai Hit environmental chamber
- Wide range of objectives suitable for widefield and TIRF microscopy
- Controlled by Elements acquisition software (Nikon), including JOBS software module and AI denoising software
17. Ultramicroscope Blaze (LaVision/Miltenyi BioTec) – “Polly II“
- Automated light sheet microscope for cleared samples
- Olympus microscope stand with infinity corrected optics
- 1.1x/0.1 NA MI PLAN objective with 15.7-17.6 mm WD, depending on the refractive index of the imaging solution with two dipping caps for organic/aqueous buffers
- 4x/0.35 MI PLAN objective with 16 mm WD with dipping cap for organic solvents
- 488, 561, 640 and 785 nm lasers
- Pco.edge 4.2 sCMOS camera
- Light speed mode
- For use with fixed and cleared specimens or transparent live specimens
Workstations and software for image processing
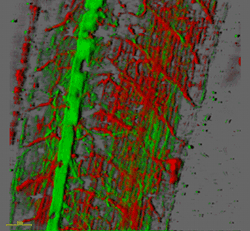
Ariel Levine, Alison North and Ali Hemmati-Brivanlou (Rockefeller University)
Windows (“QueenVic”, “CharlesDarwin”, “Bobo”, “Ginger II”, “Nick” and “Frizzle”), and Linux (“Gromit”) high-end workstations and servers are available for processing and analyzing acquired images, including 64-bit multi-processor systems with up to 512 GB of memory for processing large multidimensional datasets using 64-bit enabled software packages. Available software includes:
- Huygens and Autoquant deconvolution software – modules for deconvolving confocal, widefield, spinning disk, Airyscan, light sheet or multiphoton data;
- MetaMorph (Universal Imaging) – cell counting, tracking, measurements, kymographs;
- Imaris including Surpass, Cell and FilamentTracer modules (Bitplane) – volume rendering in 3D and 4D, surface rendering, measurements, colocalization, clipping planes, 4D tracking, filament tracing;
- Arivis Vision4D (Zeiss) – interactive 3D/4D rendering and analysis of large data sets;
- Arivis InViewR (Zeiss) – Virtual Reality station for climbing into your 3D data sets;
- Fiji/ImageJ (freely downloadable software)* – popular open source software for image processing and analysis;
- SoftWoRx (Applied Precision/ DeltaVision) – deconvolution, volume rendering, surface rendering, quantification;
- Zen confocal software (Zeiss) and Elements software (Nikon) – projections, movies, ROI measurements, intensity profiles, etc.
- AIVIA (Leica) – visualization and analysis software with state-of-the-art machine/deep learning tools
The Linux workstation “Gromit” is dedicated to deconvolving and processing images collected on the DeltaVision and OMX microscopes.
All users are urged to save their data directly to their folder on the bio-imaging server, ready for processing on the workstations. This frees up the microscopes for image acquisition by other users. To encourage this, the charge for time on the workstations is considerably lower than the microscope charges. Data on the server can be accessed directly from the user’s laboratory computers, or can be copied to external hard drives (users are responsible for bringing their own drives).
* To install ImageJ for free on your own computer, click on the following two web sites:
https://imagej.nih.gov/ij/download.html
https://www.openmicroscopy.org/bio-formats/downloads
Additional lab equipment for live cell imaging
Two cell culture incubators are present in the general laboratory area, one with CO2 and the other without (for cells in CO2-independent media). These can be used for temporary storage of specimens during live cell imaging experiments. A class II biological safety cabinet is also available for use. Please note that the center is not designed to accommodate infectious, radioactive or otherwise hazardous specimens. If you are in any doubt as to the suitability of your specimens for live cell imaging in the center, please contact Alison. Anyone who performs unauthorized experiments that place the other users’ health at risk will be banned from further use of the center.