Therapeutic Development Awards
Drug development is costly, time consuming and requires specific expertise. The Rockefeller University has established formal mechanisms to provide both dedicated funding and essential guidance for promising Rockefeller translational research projects. Recipients of Robertson therapeutic development awards have shown that fundamental knowledge, strategically applied, can de-risk projects and make them more attractive to pharmaceutical or biotech companies.
Translational grants are awarded through a highly competitive process, with streamlined application and evaluation procedures. All proposals are reviewed by an independent committee of experts drawn from the pharmaceutical, biotechnology, and life sciences investment industries. Funding is distributed according to a schedule based upon the successful completion of agreed-upon milestones.
Requests for proposals are announced via campus email.
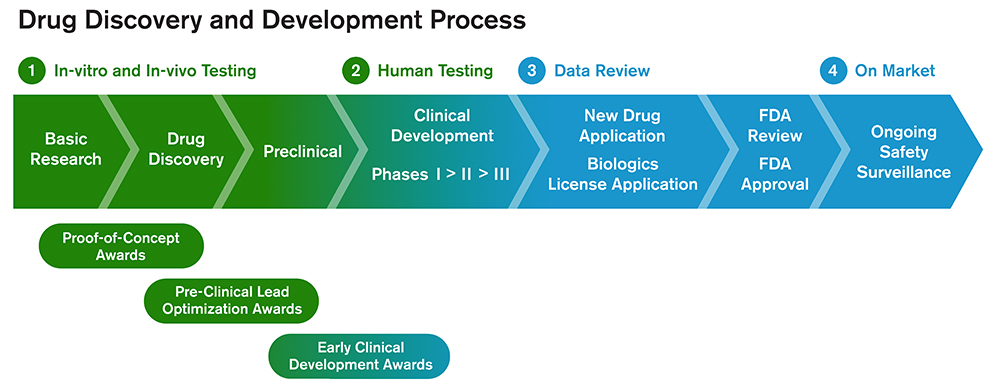
Three types of therapeutic development awards are offered.
Proof-of-Concept Awards
Early-stage Proof-of-Concept (POC) Awards support studies aimed at identifying and validating potential therapeutic targets and diagnostics. POC Awards are typically offered twice a year and are designed to jumpstart early-stage initiatives not fundable by the National Institutes of Health or other traditional granting agencies.
- Pilot POC Awards provide $25,000 to $100,000, often enabling scientists to gather the preliminary data needed to launch a project. To date, approximately $10.5 million in TDF funding has supported 223 grants.
- Advanced POC Awards provide $100,000 to $300,000, for more mature projects, funding compound screening/profiling, functional testing in cell assays, initial medicinal chemistry, non-clinical pharmacokinetics, and initial testing in pharmacological models of disease. A total of 55 grants providing nearly $11.1 million in support have been awarded.
Pre-Clinical Lead Optimization Awards
Pre-Clinical Lead Optimization (PCLO) Awards support studies to deliver high quality drug candidates as they transition toward the clinic. PCLO Awards are offered as funding permits with a target of once per year. Preference for funding is given to commercially viable projects that have intellectual property in place and a clear path towards an Investigational New Drug application (IND).
These awards range from $300,000 to $1.2 million and are intended to fund high-cost, outsourced studies which may include medicinal chemistry, in vitro metabolic profiling, safety pharmacology, pharmacokinetics analysis, and pre-clinical toxicology studies. Introduced in 2019, the PCLO grants are currently providing $3.7M in funding for eight mid-stage projects.
Early Clinical Development Awards
Early Clinical Development (ECD) Awards support studies to secure IND approval and, in some cases, advance into “first-in-human” phase 1 clinical trials. This may include funding for scale-up of drug substance under good manufacturing practices (GMP), toxicology studies conducted under good laboratory practice (GLP) conditions, clinical formulations, and clinical trial expenses. These larger awards are typically offered every other year.
These awards provide up to $2.5 million over two years for later-stage projects. To date, 10 ECD grants totaling $23.4 million have supported projects developing treatments for solid and metastatic cancers, infectious disease (HIV and Hepatitis B), heart attack, and autoimmunity. Four of these programs are currently in clinical trials.