New technique reveals a role for histones in cell division
Proteins known as histones give structure to DNA, which coils around them like string on spools. But as is so often the case in biology, it turns out there is more to these structures than meets the eye. Scientists already know histones play a part in controlling the expression of genes, and more recently they have accumulated evidence that certain aspects of cell division depend on these proteins. But this last suspicion has proven difficult to test.
Now a new technique developed at Rockefeller University in Hironori Funabiki’s Laboratory of Chromosome and Cell Biology allows researchers to examine histones’ role in crucial cell division processes that revolve around DNA, such as the segregation of chromosomes and the construction of the cell’s nucleus.
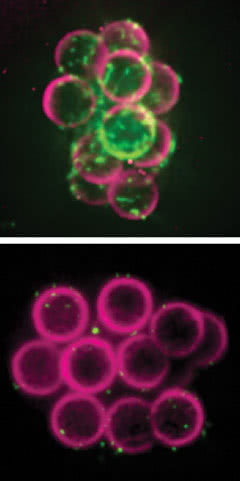
Building on beads: Researchers found that beads covered with histones and DNA (top) attracted a protein called lamin B3 (green) that supports the membrane around a new cell’s nucleus. Beads with only DNA (bottom) did not attract lamins.
“Manipulating the genes that code for histones — the typical approach for this sort of research — usually causes massive changes within the cell. But because histones themselves regulate gene expression, the reasons for these changes are nearly impossible to interpret,” Funabiki says. “We have addressed this problem by developing a new way to study histones that sidesteps this problem, and we have used it to explore these proteins’ role in organizing large-scale, DNA-based structures within the cell.”
Histones without DNA are like an instruction manual without words, so these experiments, led by research associate Christian Zierhut, focused on the two together, which form structures known as nucleosomes. Their work revealed nucleosomes must be present in order for the mitotic spindle, the cellular machine responsible for dividing up chromosomes during cell division, to form. Additionally, nucleosomes proved necessary for some key aspects in the formation of the nuclear envelope that cordons off the cell’s control center.
To experiment with histones, Zierhut and his colleagues turned to frog eggs, a model system ideally suited to studies of cell division. Scientists have long known it is possible to purify the fluid, or cytosol, from inside the eggs, and then, by adding DNA, induce almost every event in a cell’s life cycle. Because these events play out outside the cell, they are much more accessible. What’s more, the eggs are transcriptionally silent, meaning no genes are expressed. As a result, the researchers did not need to deal with confusing downstream effects. However, these eggs carry large stockpiles of histones, making histone manipulation a daunting task.
“Just as manipulating histone genes is not a realistic approach, neither is getting rid of them altogether, because histones, like DNA, are essential for viability. This has been a major hurdle for histone research, and one we managed to overcome,” Zierhut says. “We found a way to generate an egg extract free from essential histones, an accomplishment once thought not to be possible.”
By thus interfering with nucleosome formation in the extract, the researchers could test what happened when they added naked DNA or nucleosomes with altered histones.
In experiments described in the July issue of Nature Structural Molecular Biology, they added DNA alone to the frog-egg extract, and saw that the mitotic spindles that drive cell division did not form. But when they added nucleosomes, the spindles formed. Using similar experiments, the researchers examined histones’ role in other aspects of this process — with mixed results. They found membrane proteins, important for the formation of the nucleus in the daughter cells, were attracted to the naked DNA. However, histones proved necessary for the formation of a support structure called the lamina. The experiments also showed an important role for them in the formation of nuclear pore complexes, which act as selective gates into and out of the nucleus. In this case, histones appear to help initiate the process by recruiting two other molecules, RCC1 and ELYS.
“The requirement for the presence of histones may help the cell control where the mitotic spindle and the nuclear envelope form,” Funabiki says. “This is particularly important for large cells that need to make sure they are forming these structures in the right spot, on or around their own histone-containing chromosomes. Without this requirement, the cells may risk establishing a nuclear envelope around viral DNA, for example, or forming nuclear pores on other intracellular membrane structures not connected to DNA. This way the cell ensures only the right set of genetic instructions can be preserved, accessed and propagated over generations.”
 |
Nature Structural & Molecular Biology 21, 617–625 (July 2014) Nucleosomal regulation of chromatin composition and nuclear assembly revealed by histone depletion Christian Zierhut, Christopher Jenness, Hiroshi Kimura and Hironori Funabiki |