Rockefeller grants commercial license for the development of monoclonal antibodies for treatment of COVID-19
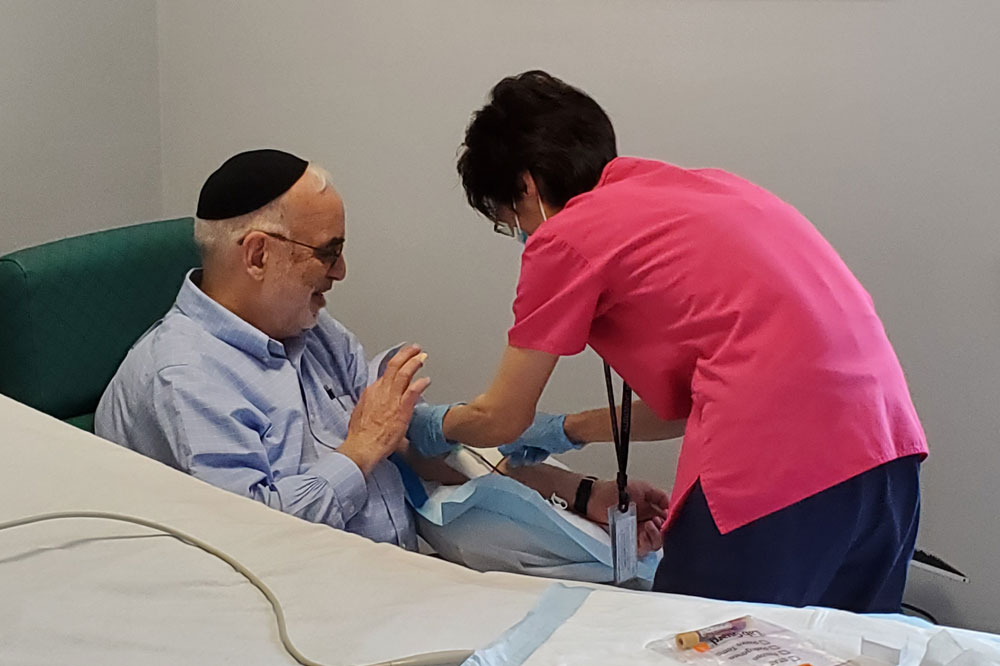
A study volunteer who has recovered from COVID-19 donates blood at the Rockefeller University Hospital.
With promising results from preclinical studies and with human trials now underway, Rockefeller has taken the next step with its novel COVID-19 treatment. The university has entered a licensing agreement with a global pharmaceutical company to advance development of a drug based on two monoclonal antibodies discovered at the university, with the goal to enable affordable, worldwide distribution.
Scientists in Michel C. Nussenzweig’s laboratory discovered potent monoclonal antibodies, which they characterized together with researchers in the labs of Paul Bieniasz and Charles M. Rice at Rockefeller, collaborating with Pamela Bjorkman’s group at Caltech. The antibodies are being used to design a new treatment to prevent people with early stages of COVID-19 from developing severe disease. Monoclonal antibody therapies can be life-saving and are urgently needed as hospitals continue to be inundated by repeated surges of infection, and mass vaccinations are still several months away.
“Thanks to the generous volunteers who donated blood plasma and the many researchers bringing their expertise together, we have been able to identify and optimize two potent antibodies that show high potential for preventing or treating COVID-19,” says Nussenzweig, the Zanvil A. Cohn and Ralph M. Steinman Professor and head of the Laboratory of Molecular Immunology at Rockefeller.
Rockefeller’s monoclonal antibodies are highly potent in blocking the SARS-CoV-2 spike protein and neutralizing the virus, and have been engineered to last longer in the bloodstream than typical antibodies. Preclinical data suggest they could effectively neutralize the virus at a low dose, which would enable large patient populations to be treated, possibly via injections as opposed to blood infusions—advantages that would help expand access to low- and middle-income countries and communities with limited healthcare resources.
Clinical trials to test the treatment’s safety and assess its optimal dosing began at Rockefeller University Hospital in January.
The treatment may also help mobilize the response to new variants of SARS-CoV-2 recently discovered in the United Kingdom, South Africa, and Brazil as well as other variants yet to emerge. As the two antibodies block the virus in slightly different ways, researchers believe their combination will reduce the virus’s ability to mutate and develop resistance to the therapy. Moreover, recent lab studies show the combination of two antibodies are active against SARS-CoV-2’s concerning mutations and major emerging variants.
Under the agreement, the company, Bristol Myers Squibb, has obtained rights to develop, manufacture, and commercialize Rockefeller’s monoclonal antibodies for the treatment of COVID-19.
Learn more about the agreement and Rockefeller’s broad range of COVID-19 research.