Bio-Imaging Resource Center
Welcome to the Frits and Rita Markus Bio-Imaging Resource Center (RRID:SCR_017791) at The Rockefeller University! We provide members of the University and their visitors with a wide spectrum of optical microscopy equipment and extensive training in its use. The BIRC was established in early 2001 and is housed in the Bronk Laboratory (DWB 201-203).We are keen to receive input from all Rockefeller University researchers to enable us to address as many of their needs as possible. The staff of the center actively develop and maintain a wide range of state-of-the-art microscopes and advise users on which instrument is most suitable for their needs. Consultation on sample preparation and immunolabeling procedures is also provided and aliquots of certain reagents are available for testing. Researchers are trained to use the microscopes and image acquisition software themselves, with staff assistance when necessary to ensure collection of high quality images. Data can then be processed in the neighboring image processing suite for export into the required format. Research can also be performed on a collaborative basis with the staff of the center.
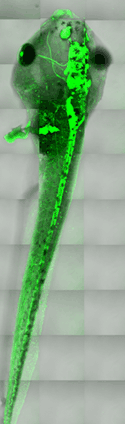
Image:
Live Xenopus tadpole expressing a transgenic GFP marker for the nervous system. 30 tiled stacks were collected using a Zeiss LSM 510 confocal microscope, allowing the entire embryo to be visualized.
Ignacio Munoz-sanjuan, Alison North and Ali Hemmati-Brivanlou (Rockefeller University), in collaboration with Nick Marsh-Armstrong (Johns Hopkins)